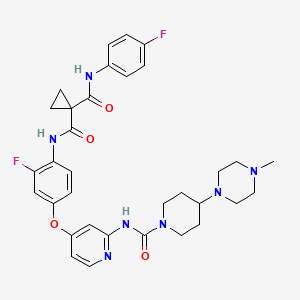
Golvatinib
E-7050, cas 928037-13-2
1-N’-[2-fluoro-4-[2-[[4-(4-methylpiperazin-1-yl)piperidine-1-carbonyl]amino]pyridin-4-yl]oxyphenyl]-1-N-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
| Molecular Formula: | C33H37F2N7O4 |
|---|---|
| Molecular Weight: | 633.701 g/mol |
-
N’-[2-fluoro-4-[2-[
[4-(4-methylpiperaz in-1-yl)piperidine- 1-carbonyl]amino]py ridin-4-yl]oxypheny l]-N-(4-fluoropheny l)cyclopropane-1,1- dicarboxamide UNII:516Z3YP58E -
Originator Eisai Co Ltd
- Class Amides; Antineoplastics; Cyclopropanes; Fluorobenzenes; Piperazines; Piperidines; Pyridines; Small molecules
- Mechanism of Action Angiogenesis inhibitors; Proto oncogene protein c met inhibitors; Vascular endothelial growth factor receptor-2 antagonists
- Discontinued Gastric cancer; Glioblastoma; Head and neck cancer; Liver cancer; Malignant melanoma; Solid tumours
- 15 Nov 2013Eisai completes enrolment in its phase Ib/II trial for Head and neck cancer (second-line combination therapy, late-stage disease) in USA, United Kingdom, South Korea & Ukraine (NCT01332266)
- 14 Nov 2013Phase-I/II clinical trials in liver cancer (first-line combination therapy, late-stage disease) in Italy & Ukraine (PO)
- 01 Jul 2013Eisai completes a phase I trial in Solid tumours in Japan (NCT01428141)
Golvatinib is an orally bioavailable dual kinase inhibitor of c-Met (hepatocyte growth factor receptor) and VEGFR-2 (vascular endothelial growth factor receptor-2) tyrosinekinases with potential antineoplastic activity. c-Met/VEGFR kinase inhibitor E7050 binds to and inhibits the activities of both c-Met and VEGFR-2, which may inhibit tumor cell growth and survival of tumor cells that overexpress these receptor tyrosine kinases. c-Met and VEGFR-2 are upregulated in a variety of tumor cell types and play important roles in tumor cell growth, migration and angiogenesis.
Method for producing a phenoxy pyridine derivative (3)
The present invention, hepatocyte growth factor receptor (Hepatocyte growth factor receptor; hereinafter, abbreviated as “HGFR”) inhibitory action, antitumor action, anti-tumor agents with such angiogenesis inhibitory activity and cancer metastasis inhibitory action, a cancer metastasis suppressing the method for producing a useful phenoxy pyridine derivatives as agents.
Patent Document 1 has a HGFR inhibitory activity, anti-tumor agents, useful phenoxy pyridine derivative as an angiogenesis inhibitor or cancer metastasis inhibitor has been disclosed.
(In the formula, R 1, .R 2 and R 3 means such as 3-10 membered non-aromatic heterocyclic group, .R 4, R 5, R 6 and R 7 which represents a hydrogen atom, same or different, a hydrogen atom, a halogen atom, .R 8 to mean a C 1-6 alkyl group, .R 9 to mean a hydrogen atom or the like is and 3-10 membered non-aromatic heterocyclic group meaning .n is .X to mean 1 to 2 integer, it refers to a group or a nitrogen atom represented by the formula -CH =.)
As a method for producing the phenoxy pyridine derivative, to the Example 48 of Patent Document 1, N, N-dimethylformamide, triethylamine and benzotriazol-1-yloxytris (dimethylamino) or lower in the presence of a phosphonium hexafluorophosphate discloses that perform the reaction.
Patent Document 2, as a manufacturing method suitable for industrial mass synthesis of the phenoxy pyridine derivative in the presence a condensing agent, production method of reacting an aniline derivative with a carboxylic acid derivative.
(In the formula, R 1, is .R 2, R 3, R 4 and R 5, which means such good azetidin-1-yl group which may have a substituent, the same or different and each represents a hydrogen atom or fluorine It refers to an atom .R 6 means a hydrogen atom or a fluorine atom.)
Patent Document 3, another manufacturing method of the phenoxy pyridine derivative, there is disclosed the manufacturing method shown in the following scheme.
(In the formula, R 1 means a 4- (4-methylpiperazin-1-yl) piperidin-1-yl group or a 3-hydroxy-1-yl group .R 2, R 3, R 4 and R 5 are the same or different, represents a hydrogen atom or a fluorine atom. However, among R 2, R 3, R 4 and R 5, 2 or 3 is a hydrogen atom .R 6 is a hydrogen atom or .R 7 to mean a fluorine atom, .Ar which means a protecting group for the amino group means a phenyl group.)
International Publication No. WO 2007/023768 International Publication No. WO 2008/026577 International Publication No. WO 2009/104520
[Formula
1 H-NMR Spectrum (DMSO-d 6) .Delta. (Ppm): 1.22-1.33 (2H, m), 1.54-1.63 (4H, m), 1.68-1.78 (2H, m), 2.12 (3H , S), 2.12-2.40 (5H, m), 2.40-2.60 (4H, m), 2.68-2.78 (2H, m), 4.06-4.14 (2H, t, J = 8 Hz), 7.22 (2H, m), 6.60 (1H, dd, J = 2.4 Hz, 5.6 Hz), 7.00 (1 H, dd, J = 2.4 Hz, 11.2 Hz), 7.40 (1 H, s), 7.61 (2 H, dd, J = 5.2 Hz, 8 Hz), 7.93 J = 8.8 Hz), 8.13 (1 H, d, J = 5.6 Hz), 9.21 (1 H, s), 9.90 (1 H, brs), 10.55 (1 H, brs).
///////////////Golvatinib, phase 2, ゴルバチニブ ,
CN1CCN(CC1)C2CCN(CC2)C(=O)NC3=NC=CC(=C3)OC4=CC(=C(C=C4)NC(=O)C5(CC5)C(=O)NC6=CC=C(C=C6)F)F