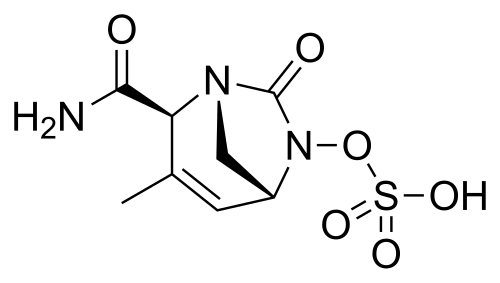
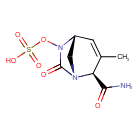
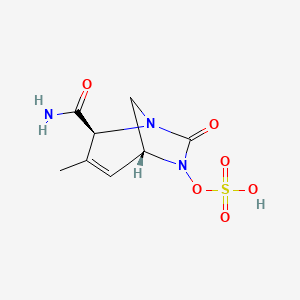
Durlobactam
CAS 1467829-71-5
WeightAverage: 277.25
Monoisotopic: 277.03685626
Chemical FormulaC8H11N3O6S
| Ingredient | UNII | CAS | InChI Key |
|---|---|---|---|
| Durlobactam sodium | F78MDZ9CW9 | 1467157-21-6 | WHHNOICWPZIYKI-IBTYICNHSA-M |
FDA 5/23/2023, Xacduro, To treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia caused by susceptible isolates of Acinetobacter baumannii-calcoaceticus complex
Press Release
Drug Trials Snapshots
(2S,5R)-2-CARBAMOYL-3-METHYL-7-OXO-1,6-DIAZABICYCLO(3.2.1)OCT-3-EN-6-YL SULFATE
SULFURIC ACID, MONO((2S,5R)-2-(AMINOCARBONYL)-3-METHYL-7-OXO-1,6-DIAZABICYCLO(3.2.1)OCT-3-EN-6-YL) ESTER
ETX 2514, ETX-2514, ETX2514, WHO 10824
Durlobactam is a non-beta-lactam, beta-lactamase inhibitor used to treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia.
Durlobactam is a beta-lactamase inhibitor used in combination with sulbactam to treat susceptible strains of bacteria in the genus Acinetobacter[1] It is an analog of avibactam.
The combination therapy sulbactam/durlobactam was approved for medical use in the United States in May 2023.[1]
PATENT
| Patent Number | Pediatric Extension | Approved | Expires (estimated) | |
|---|---|---|---|---|
| US9309245 | No | 2016-04-12 | 2033-04-02 | |
| US9623014 | No | 2017-04-18 | 2033-04-02 | |
| US9968593 | No | 2018-05-15 | 2035-11-17 | |
| US10376499 | No | 2019-08-13 | 2035-11-17 |
SYN
.org/10.1021/acs.jmedchem.4c02079
J. Med. Chem. 2025, 68, 2147−2182
durlobactam (1) is a copackaged antibiotic combination
being developed by Entasis Therapeutics for the treatment of
infections caused by Acinetobacter baumannii-calcoaceticus.
13,14
Entasis Therapeutics obtained the worldwide development
rights for durlobactam (1) from AstraZeneca in 2015.13 The
drug combination was approved by the USFDA in 2023 for use
in patients 18 years of age and older as an intravenous infusion.13
Acinetobacter baumannii is a critical bacterial pathogen that has
become highly resistant to various β-lactam antibiotics for
Gram-negative infections, including penicillin.15,16 The inventors targeted β-lactam resistance via coadministration of a
β-lactamase inhibitor to restore the activity of β-lactam
antibiotics. Sulbactam is a β-lactam antibiotic that inhibits
penicillin binding proteins (PBP 1 and 3) essential for cell wall
synthesis. Durlobactam is a β-lactamase inhibitor that protects
sulbactam from degradation by Ambler class A, C, and D serine
β-lactamases produced byAcinetobacter baumannii-calcoaceticus.
Durlobactam binds covalently with these β-lactamases by
carbamoylating the active site serines, thus safeguarding
sulbactam from enzymatic degradation.17,18 The covalent
bond between durlobactam and the active site serine isreversible
due to the ability of sulfated amine of durlobactam to recyclize
back into urea. This allows durlobactam to exchange from one
enzyme molecule to another via a mechanism known as
acylation exchange
(13) Keam, S. J. Sulbactam/Durlobactam: first approval. Drugs 2023,
83, 1245−1252.
(14) El-Ghali, A.; Kunz Coyne, A. J.;Caniff, K.; Bleick,C.; Rybak, M. J.
Sulbactam-durlobactam: a novel β-lactam-β-lactamase inhibitor
combination targeting carbapenem-resistant Acinetobacter baumannii
infections. Pharmacotherapy 2023, 43, 502−513.
(15) O’Donnell, J.; Tanudra, A.; Chen, A.; Miller, A. A.; McLeod, S.
M.; Tommasi, R. In vitro pharmacokinetics/pharmacodynamics of the
β-lactamase inhibitor, durlobactam, in combination with sulbactam
against Acinetobacter baumannii-calcoaceticus complex. Antimicrob.
Agents Chemother. 2024, 68, e00312-23.
(16) Arya, R.; Goldner, B. S.; Shorr, A. F. Novel agents in development
for multidrug-resistant Gram-negative infections: potential new options
facing multiple challenges. Curr. Opin. Infect. Dis. 2022, 35, 589−594.
(17) Shapiro, A. B.; Moussa, S. H.; McLeod, S. M.; Durand-Réville, T.;
Miller, A. A. Durlobactam, a new diazabicyclooctane β-lactamase
inhibitor for the treatment of Acinetobacter infections in combination
with Sulbactam. Front. Microbiol. 2021, 12, No. 709974.
(18) Iyer, R.; Moussa, S. H.; Durand-Reville, T. F.; Tommasi, R.;
Miller, A. Acinetobacter baumannii OmpA is a selective antibiotic
permeant porin. ACS Infect. Dis. 2018, 4, 373−381.


The route below was chosen as it was demonstrated on a
multikilogram scale (Scheme 1), although some reagents (e.g.,
triphosgene) are not typical for large-scale manufacturing.20,22
The synthesis commenced with the condensation of glyoxylic
acid monohydrate (1.1) with (S)-tert-butylsulfinamide (1.2) to
generate a solution of 2-(tert-butylsulfinylimino)acetic acid 1.3.
In parallel, commercially available trans-crotyl alcohol (1.4) was
treated with diboronic acid (1.5) in the presence of a palladium
catalyst to produce a solution of crotylboronic acid 1.6. These
two solutions were mixed to afford chiral α-amino acid 1.7 in
58% overall yield. Diastereo- and enantioselectivity were not
reported for the transformation.
Conversion of 1.7 into durlobactam sodium (1) is described
in Scheme 2. First, the carboxylic acid 1.7 was converted to an
amide and the sulfinamide was removed to afford amino amide
1.8 as an HCl salt. The primary amine in 1.8 was subsequently
alkylated with allyl bromide (1.9) and the resulting allyl amine
was protected with Boc anhydride to provide olefin metathesis
precursor 1.10. Bisolefin 1.10 was then subjected to Grubbs
first-generation catalyst (Grubbs-I) to generate a tetrahydropyridine precursor, which participated in a one-pot nitroso-ene
reaction with N-Boc hydroxylamine (1.11) to produce allyl
hydroxylamine 1.12 in 61% overall yield. This key transformation efficiently installed the amine stereocenter required
for formation of the bridged urea. Next, the hydroxyl moiety in
1.12 was protected as the TBS ether and the two Boc groups
were removed with ZnBr2 to unveil bis-amine 1.13. The
intramolecular urea formation was accomplished by the
treatment with triphosgene to generate diazabicyclooctene
1.14 in 50% yield over 3 steps. The TBS ether was then
removed, and the hydroxyl urea intermediate was treated with
sulfur trioxide-pyridine complex and tetrabutylammonium
hydrogen sulfate to afford durlobactam tetrabutylammonium
salt 1.15. Finally, tetrabutylammonium durlobactam 1.15 was
converted to a calcium salt and subsequently to the targeted
sodium salt providing 1. The authors mentioned that the salt
formations were required to improve the purity of the final API
(>99%), however, the yields of these steps were not reported.22
(19) McGuire, H.; Bist, S.; Bifulco, N.; Zhao, L.; Wu, Y.; Huynh, H.;
Xiong, H.; Comita-Prevoir, J.; Dussault, D.; Geng, B.; et al. Preparation
of oxodiazabicyclooctenyl hydrogen sulfate derivatives for use as betalactamase inhibitors. WO 2013150296, 2013.
(20) Basarab, G. S.; Moss, B.; Comita-Prevoir, J.; Durand-Reville, T.
F.; Gauthier, L.; O’Donnell, J.; Romero, J.; Tommasi, R.; Verheijen, J.
C.; Wu, F.; et al. Preparation of substituted 2-(1,6-diazabicyclo[3.2.1]-
oct-3-en-6-yloxy)acetates as beta-lactamase inhibitors. WO
2018053215, 2018.
(21) Durand-Reville, T. F.;Comita-Prevoir, J.; Zhang, J.; Wu, X.; MayDracka, T. L.; Romero, J. A. C.; Wu, F.; Chen, A.; Shapiro, A. B.; Carter,
N. M.; et al. Discovery of an orally available diazabicyclooctane
inhibitor (ETX0282) of class A, C, and D serine β-lactamases. J. Med.
Chem. 2020, 63, 12511−12525.
(22) Durand-Reville, T. F.; Wu, F.; Liao, X.; Wang, X.; Zhang, S.
Preparation of Durlobactam crystalline forms. WO 2023206580, 2023
syn
https://www.mdpi.com/1424-8247/15/3/384
Synthesis of Durlobactam
Chemically, durlobactam is [(2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diazabicyclo [3.2.1] oct-3-en-6-yl] hydrogen sulfate which can be prepared from the key intermediate hydroxyurea 6-hydroxy-3-methyl-7-oxo-1,6-diaza-bicyclo [3.2.1] oct-3-ene-2-carboxylic acid amide I, which is the structural isomer of III prepared to synthetize ETX-1317 [101]. Then, according to Scheme 15, compound 1 obtained in the synthesis of III (Scheme 14) was reacted with penta-1,3-diene in place of isoprene, and, by an aza-Diels−Alder reaction, compound 2 was obtained.
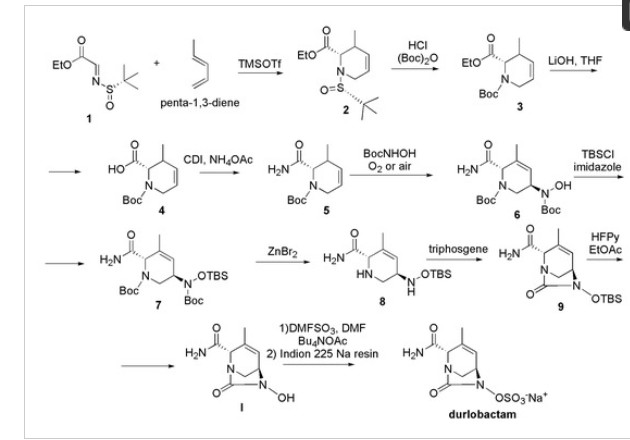
Scheme 15. Synthesis of durlobactam (C8H11N3O6S, MW = 277.36, IUPAC name, [(2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diazabicyclo [3.2.1] oct-3-en-6-yl] hydrogen sulphate.
Compound 2 underwent deprotection of the tert-butyl sulfinyl group to afford 3, subsequently Boc protected, to give compound 4. The saponification of the ester followed by amide coupling using ammonium acetate afforded compound 5. The reaction of alkene 5 with N-Boc-hydroxylamine in the presence of oxygen or air gave the desired compound 6 in a single step. Compound 6 was then protected with TBS group, using TBSCl to afford 7, which was Boc deprotected using zinc bromide obtaining compound 8. Cyclization of the diamine 8 with tri-phosgene provided the corresponding cyclic urea 9, which was TBS deprotected with HFPy to give the key intermediate I. This compound was then immediately sulfated with the DMF:SO3 complex to obtain the sulfate, which was isolated as its tetrabutylammonium salt 10 by reacting with tetrabutylammonium acetate. The tetrabutylammonium salt was converted to durlobactam in the form of sodium salt by passing 10 through a column filled with Indion 225 sodium resin.
REF
https://sioc-journal.cn/Jwk_yjhx/EN/abstract/abstract350784.shtml
References
- ^ Jump up to:a b “FDA Approves New Treatment for Pneumonia Caused by Certain Difficult-to-Treat Bacteria”. U.S. Food and Drug Administration (Press release). 24 May 2023. Retrieved 24 May 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Further reading
- Shapiro AB, Moussa SH, McLeod SM, Durand-Réville T, Miller AA (2021). “Durlobactam, a New Diazabicyclooctane β-Lactamase Inhibitor for the Treatment of Acinetobacter Infections in Combination With Sulbactam”. Frontiers in Microbiology. 12: 709974. doi:10.3389/fmicb.2021.709974. PMC 8328114. PMID 34349751.
- Papp-Wallace KM, McLeod SM, Miller AA (May 2023). “Durlobactam, a Broad-Spectrum Serine β-lactamase Inhibitor, Restores Sulbactam Activity Against Acinetobacter Species”. Clinical Infectious Diseases. 76 (Supplement_2): S194 – S201. doi:10.1093/cid/ciad095. PMC 10150275. PMID 37125470.
| Clinical data | |
|---|---|
| Other names | ETX2514 |
| Routes of administration | Intravenous |
| Drug class | Antibacterial, beta-lactamase inhibitor |
| ATC code | None |
| Legal status | |
| Legal status | US: ℞-only co-packaged with sulbactam |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 1467829-71-5 |
| PubChem CID | 89851852 |
| DrugBank | DB16704DBSALT003190 |
| ChemSpider | 5761778471060725 |
| UNII | PSA33KO9WAF78MDZ9CW9 |
| KEGG | D11591D11592 |
| ChEMBL | ChEMBL4298137ChEMBL4297378 |
| Chemical and physical data | |
| Formula | C8H11N3O6S |
| Molar mass | 277.25 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
/////////Durlobactam, Xacduro, FDA 2023, APPROVED 2023, ETX 2514, ETX-2514, ETX2514, WHO 10824
Syn
European Journal of Medicinal Chemistry 291 (2025) 117643
Durlobactam, developed by Entasis Therapeutics, is a novel β-lactamase inhibitor designed to combat multidrug-resistant (MDR) Acinetobacter baumannii infections [83]. It is co-formulated with sulbactam, a
β-lactam antibiotic, and marketed under the brand name XACDURO. In 2024, the NMPA approved XACDURO for the treatment of hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by susceptible isolates of Acinetobacter baumannii-calcoaceticus complex in adults [84]. Durlobactam inhibits a broad spectrum of β-lactamases, including class A, C, and D enzymes, which are commonly produced by A. baumannii. By protecting sulbactam from enzymatic degradation, it restores sulbactam’s antibacterial activity against these resistant pathogens. The
clinical efficacy of sulbactam-durlobactam was demonstrated in the PhaseIII ATTACK trial (NCT03894046), a randomized, active-controlled study comparing sulbactam-durlobactam to colistin in patients with infections caused by carbapenem-resistant A. baumannii [85]. In this trial, the primary efficacy endpoint was achieved. It demonstrated non-inferiority in terms of 28-day all-cause mortality. The mortality rate in the sulbactam – durlobactam group was 19.0 %, while that in the colistin group reached 32.3 %. Moreover, the incidence of nephrotoxicity was remarkably lower in the sulbactam-durlobactam
group. From the perspective of toxicity, sulbactam-durlobactam was typically well-tolerated by the subjects. The most common adverse reactions included liver function test abnormalities, diarrhea, and hypokalemia. Notably, the incidence of nephrotoxicity was lower compared to colistin, highlighting a more favorable safety profile. The approval of XACDURO provides a targeted therapeutic option for managing severe infections caused by MDR A. baumannii, addressing a critical need in the
treatment of these challenging pathogens [86–88].
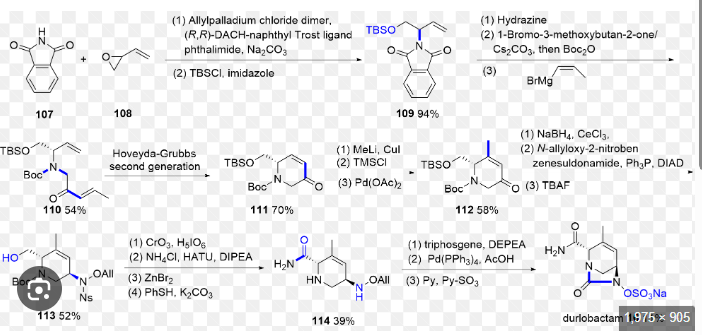
The synthetic route of Durlobactam, shown in Scheme 20, commences with a Grignard substitution between Durl-001 and Durl-002, affording Durl-003 [89]. This intermediate undergoes Diels-Alder
cyclization to form Durl-004, followed by reduction to Durl-005. Mitsunobu reaction of Durl-005 generates Durl-006, which is subjected to sequential deprotections yielding Durl-007 and subsequently Durl-008. Amidation of Durl-008 produces Durl-009, followed by TBAF-mediated deprotection to afford Durl-010. Oxidation of Durl-010Ngives carboxylic acid Durl-011, which undergoes amidation to form
Durl-012. Palladium-catalyzed coupling of Durl-012 produces Durl-013, with final ion exchange affording Durlobactam.
83-89
[83] A.B. Shapiro, S.H. Moussa, S.M. McLeod, T. Durand-R´ eville, A.A. Miller,
Durlobactam, a new diazabicyclooctane β-Lactamase inhibitor for the treatment of
acinetobacter infections in combination with sulbactam, Front. Microbiol. 12
(2021) 709974.
[84] G. Granata, F. Taglietti, F. Schiavone, N. Petrosillo, Durlobactam in the treatment
of multidrug-resistant Acinetobacter baumannii infections: a systematic review, J. Clin. Med. 11 (2022) 3258.
[85] K.M. Papp-Wallace, S.M. McLeod, A.A. Miller, Durlobactam, a broad-spectrum
serine β-lactamase inhibitor, restores sulbactam activity against acinetobacter
species, Clin. Infect. Dis. 76 (2023) S194–s201.
[86] Sulbactam and Durlobactam, Drugs and Lactation Database (Lactmed®), National
Institute of Child Health and Human Development, Bethesda (MD), 2006.
[87] S.J. Keam, Sulbactam/durlobactam: first approval, Drugs 83 (2023) 1245–1252.
[88] Y. Fu, T.E. Asempa, J.L. Kuti, Unraveling sulbactam-durlobactam: insights into its
role in combating infections caused by Acinetobacter baumannii, Expert Rev. Anti
Infect. Ther. 23 (2024) 1–12.
[89] H. McGuire, S. Bist, N. Bifulco, L. Zhao, Y. Wu, H. Huynh, H. Xiong, J. Comita-
Prevoir, D. Dussault, B. Geng, B. Chen, T. Durand-Reville, S. Guler, Preparation of
Oxodiazabicyclooctenyl Hydrogen Sulfate Derivatives for Use as beta-lactamase
Inhibitors, 2013 US9623014B2.















