
Zilucoplan
CAS 1841136-73-9
YG391PK0CC, RA101495, WHO 10602
3562 g/mol, C172H278N24O55
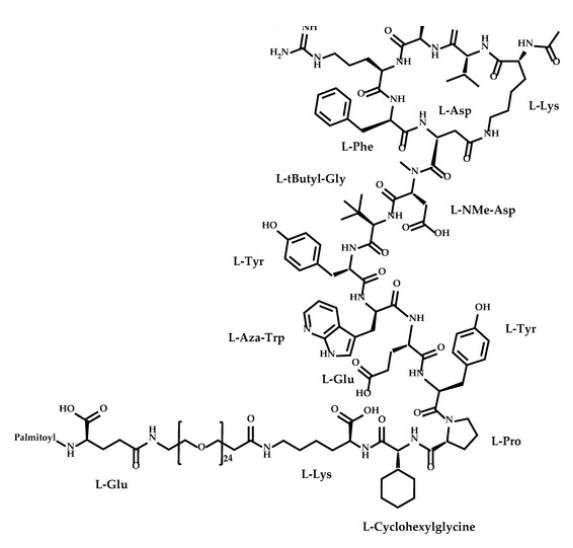
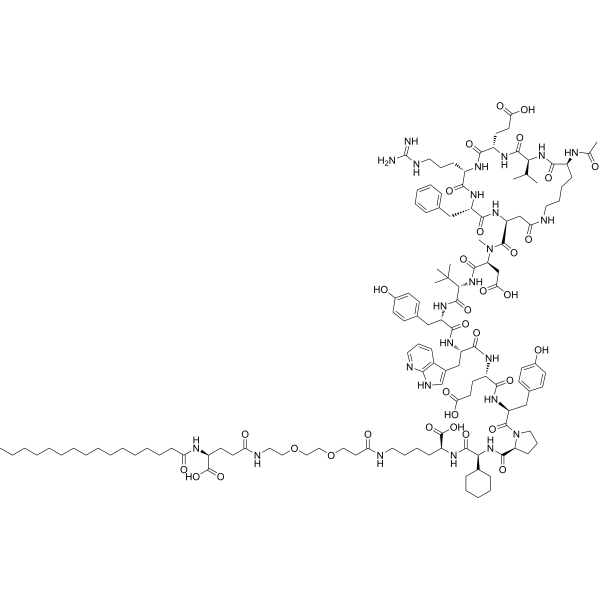
Zilucoplan lso designated as RA101495, is the active principle of Zilbrysq®, commercialized by UCB Pharma S.A. It is a 3.5 kDa synthetic macrocyclic peptide composed of 15 amino acid residues, including four unnatural amino acids [27]. The amino acid residues composition is: L-Lys, L-Val, L-Glu, L-Arg, L-Phe, L-Asp, L-L-NMe-Asp, L-tButyl-Gly, L-Tyr, L-7-aza-Trp, L-Glu, L-Tyr, L-Pro, L-Cyclohexyl-Gly, and L-Lys.
N2-ACETYL-L-LYSYL-L-VALYL-L-.ALPHA.-GLUTAMYL-L-ARGINYL-L-PHENYLALANYL-L-.ALPHA.-ASPARTYL-N-METHYL-L-.ALPHA.-ASPARTYL-3-METHYL-L-VALYL-L-TYROSYL-3-(1H-PYRROLO(2,3-B)PYRIDIN-3-YL)-L-ALANYL-L-.ALPHA.-GLUTAMYL-L-TYROSYL-L-PROLYL-(2S)-2-CYCLOHEXYLGLYCYL-N6-(3
POLY(OXY-1,2-ETHANEDIYL), ALPHA-(2-(((4S)-4-CARBOXY-1-OXO-4-(1-OXOHEXADECYL)BUTYL)AMINO)ETHYL)-OMEGA-HYDROXY-, 15-ETHER WITH N-ACETYL-L-LYSYL-L-VALYL-L-ALPHA-GLUTAMYL-L-ARGINYL-L-PHENYLALANYL-L-ALPHA-ASPARTYL-N-METHYL-L-ALPHA-ASPARTYL-3-METHYL-
(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,5S,8S,11S,14S,22S)-22-acetamido-11-benzyl-8-(3-carbamimidamidopropyl)-5-(2-carboxyethyl)-3,6,9,12,16,23-hexaoxo-2-propan-2-yl-1,4,7,10,13,17-hexazacyclotricosane-14-carbonyl]-methylamino]-3-carboxypropanoyl]amino]-3,3-dimethylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-(1H-pyrrolo[2,3-b]pyridin-3-yl)propanoyl]amino]-4-carboxybutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]pyrrolidine-2-carbonyl]amino]-2-cyclohexylacetyl]amino]-6-[3-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[[(4S)-4-carboxy-4-(hexadecanoylamino)butanoyl]amino]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]propanoylamino]hexanoic acid
| Ingredient | UNII | CAS | InChI Key |
|---|---|---|---|
| Zilucoplan Sodium | Not Available | Not Available | FUSMWKLQHKXKHI-WHKBRXDJSA-J |
FDA 10/17/2023, Zilbrysq, To treat generalized myasthenia gravis in adults who are anti-acetylcholine receptor (AChR) antibody positive
Drug Trials Snapshot
Zilucoplan, sold under the brand name Zilbrysq, is a medication used for the treatment of generalized myasthenia gravis.[6][9][10] It is a complement inhibitor that is injected subcutaneously (under the skin).[6]
Zilucoplan is a cyclic peptide that binds to the protein complement component 5 (C5) and inhibits its cleavage into C5a and C5b.[11]
Zilucoplan was approved for medical use in the United States in October 2023,[6][12] in the European Union in December 2023,[7] and in Australia in July 2024.[1]
Zilucoplan is a 15 amino-acid, synthetic macrocyclic peptide with formula C172H278N24O55. Its sodium salt is used for the treatment of generalised myasthenia gravis (a disease that leads to muscle weakness and tiredness) in adults whose immune system produces antibodies against acetylcholine receptors. It has a role as a complement component 5 inhibitor and an immunosuppressive agent. It is a macrocycle, a homodetic cyclic peptide and a polyether. It is a conjugate acid of a zilucoplan(4-).
PATENT
| Patent Number | Pediatric Extension | Approved | Expires (estimated) | |
|---|---|---|---|---|
| US11752190 | No | 2023-09-12 | 2035-06-12 | |
| US11014965 | No | 2021-05-25 | 2035-06-12 | |
| US10435438 | No | 2019-10-08 | 2035-06-12 | |
| US10208089 | No | 2019-02-19 | 2035-06-12 | |
| US10106579 | No | 2018-10-23 | 2035-06-12 | |
| US10835574 | No | 2020-11-17 | 2035-06-12 | |
| US11535650 | No | 2022-12-27 | 2035-06-12 | |
| US10562934 | No | 2020-02-18 | 2035-06-12 | |
| US11965040 | No | 2024-04-23 | 2035-06-12 |
PAPER
https://www.mdpi.com/2813-2998/3/2/18
References
- ^ Jump up to:a b c “Zilbrysq (zilucoplan)”. Therapeutic Goods Administration (TGA). 24 September 2024. Retrieved 12 October 2024.
- ^ “Therapeutic Goods (Poisons Standard—June 2024) Instrument 2024”. Federal Register of Legislation. 30 May 2024. Retrieved 10 June 2024.
- ^ “Zilbrysq (UCB Australia Pty Ltd T/A UCB Pharma Division of UCB Australia)”. Therapeutic Goods Administration (TGA). 13 September 2024. Retrieved 15 September 2024.
- ^ “Notice: Multiple additions to the Prescription Drug List (PDL) [2024-08-13]”. Health Canada. 13 August 2024. Retrieved 15 August 2024.
- ^ “Regulatory Decision Summary for Zilbrysq”. Drug and Health Products Portal. 11 July 2024. Retrieved 27 December 2024.
- ^ Jump up to:a b c d e “Zilbrysq- zilucoplan injection, solution”. DailyMed. 19 July 2024. Retrieved 15 September 2024.
- ^ Jump up to:a b c d “Zilbrysq EPAR”. European Medicines Agency. 1 December 2023. Retrieved 11 December 2023.
- ^ “Zilbrysq Product information”. Union Register of medicinal products. 4 December 2023. Archived from the original on 11 December 2023. Retrieved 11 December 2023.
- ^ Howard JF, Kaminski HJ, Nowak RJ, Wolfe GI, Benatar MG, Ricardo A, et al. (April 2018). “RA101495, a subcutaneously administered peptide inhibitor of complement component 5 (C5) for the treatment of generalized myasthenia gravis (gMG): Phase 1 results and phase 2 design (S31. 006)”. Neurology. 90 (15 Supplement). doi:10.1212/WNL.90.15_supplement.S31.006. S2CID 56969245. Archived from the original on 22 February 2022. Retrieved 24 June 2021.
- ^ Howard JF, Vissing J, Gilhus NE, Leite MI, Utsugisawa K, Duda PW, et al. (May 2021). “Zilucoplan: An Investigational Complement C5 Inhibitor for the Treatment of Acetylcholine Receptor Autoantibody-Positive Generalized Myasthenia Gravis”. Expert Opinion on Investigational Drugs. 30 (5): 483–493. doi:10.1080/13543784.2021.1897567. hdl:11250/2770699. PMID 33792453. S2CID 232482753.
- ^ Ricardo A, Arata M, DeMarco S, Dhamnaskar K, Hammer R, Fridkis-Hareli M, et al. (2015). “Preclinical Evaluation of RA101495, a Potent Cyclic Peptide Inhibitor of C5 for the Treatment of Paroxysmal Nocturnal Hemoglobinuria”. Blood. 126 (23): 939. doi:10.1182/blood.V126.23.939.939.
- ^ “Novel Drug Approvals for 2023”. U.S. Food and Drug Administration (FDA). 22 December 2023. Archived from the original on 8 January 2023. Retrieved 27 December 2023.
- ^ Jump up to:a b “Zilbrysq: Pending EC decision”. European Medicines Agency. 15 September 2023. Archived from the original on 26 September 2023. Retrieved 24 September 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ “Zilucoplan Orphan Drug Designations and Approvals”. U.S. Food and Drug Administration (FDA). Archived from the original on 17 October 2023. Retrieved 19 October 2023.
- ^ “EU/3/22/2650: Orphan designation for the treatment of myasthenia gravis”. European Medicines Agency. 15 September 2023. Archived from the original on 29 January 2023. Retrieved 24 September 2023.
- ^ World Health Organization (2018). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 80”. WHO Drug Information. 32 (3). hdl:10665/330907.
External links
- Clinical trial number NCT04115293 for “Safety, Tolerability, and Efficacy of Zilucoplan in Subjects With Generalized Myasthenia Gravis (RAISE)” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Trade names | Zilbrysq |
| Other names | RA101495 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a624002 |
| License data | US DailyMed: Zilucoplan |
| Pregnancy category | AU: D[1] |
| Routes of administration | Subcutaneous |
| Drug class | Complement inhibitor |
| ATC code | L04AJ06 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)[2][3][1]CA: ℞-only[4][5]US: ℞-only[6]EU: Rx-only[7][8] |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 1841136-73-9 |
| PubChem CID | 133083018 |
| DrugBank | DB15636 |
| ChemSpider | 71115966 |
| UNII | YG391PK0CC |
| KEGG | D12357 |
| ChEBI | CHEBI:229659 |
| Chemical and physical data | |
| Formula | C172H278N24O55 |
| Molar mass | 3562.229 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
/////////zilucoplan, Zilbrysq, FDA 2023, APPROVALS 2023, EU 2023, EMA 2023, RA101495, RA 101495, WHO 10602














