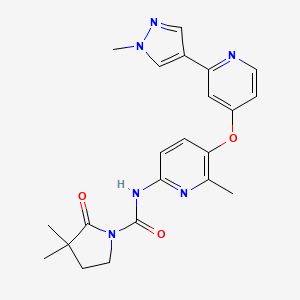
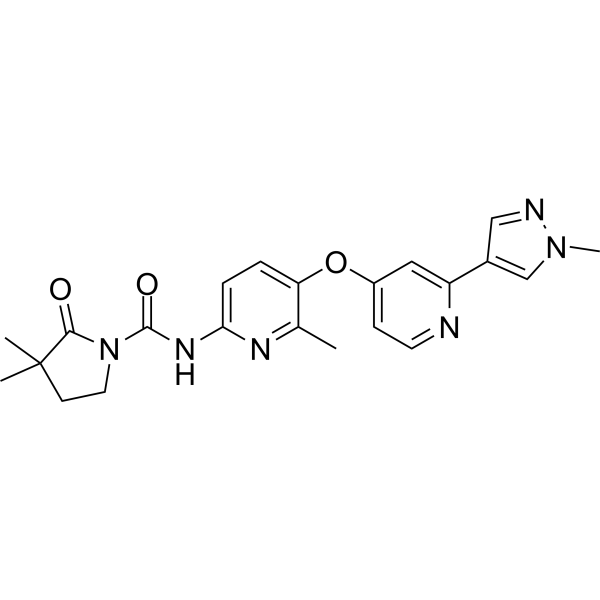
PIMICOTINIB
CAS 2253123-16-7
ABSK021
WeightAverage: 420.473
Monoisotopic: 420.190988657
Chemical FormulaC22H24N6O3
3,3-dimethyl-N-[6-methyl-5-[2-(1-methylpyrazol-4-yl)pyridin-4-yl]oxypyridin-2-yl]-2-oxopyrrolidine-1-carboxamide
CSF-1R inhibitor Pimicotinib (ABSK021) of Abbisko Therapeutics, HV1XI8HST2
Pimicotinib (ABSK021), an oral, highly potent and selective small molecule blocker of the colony-stimulating factor 1 receptor (CSF-1R) independently discovered by Abbisko Therapeutics. A number of studies have shown that blocking the CSF-1R signaling pathway could effectively modulate and change macrophage functions, and potentially treat many macrophage-dependent human diseases.[1]
Pimicotinib is under investigation in clinical trial NCT05804045 (Study of Pimicotinib (ABSK021) for Tenosynovial Giant Cell Tumor (MANEUVER)).
History
In December 2023, Abbisko Therapeutics entered into a licensing agreement for pimicotinib in all indications for China rights with Merck KGaA.[2] [3][4]
In April 2023, a global phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial designed to evaluate the safety and efficacy of pimicotinib in patients with tenosynovial giant cell tumor was started (NCT05804045).[5]
Following with pimicotinib for tenosynovial giant cell tumor treatment in phase III, pimicotinib has also entered into a phase II trial in June 2023 for cGVHD treatment in China.[6]
The U.S. Food and Drug Administration (FDA) and the Center for Drug Evaluation (CDE) of NMPA granted pimicotinib breakthrough therapy designation (BTD) for the treatment of tenosynovial giant cell tumor patients that are not amenable to surgery in January 2023 and July 2022, respectively.[7]
Research
Pimicotinib is being investigated as a treatment for tenosynovial giant cell tumor,[8][9] chronic graft-versus-host-disease (cGVHD), and pancreatic cancer.
PATENTS
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018214867&_cid=P10-MCTXKZ-70170-1
Example 41: Preparation of 3-hydroxy-3-methyl-N-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)pyridin-4-yl)oxy)pyridin-2-yl)-2-carbonylpyrrolidine-1-carboxamide
[0463]
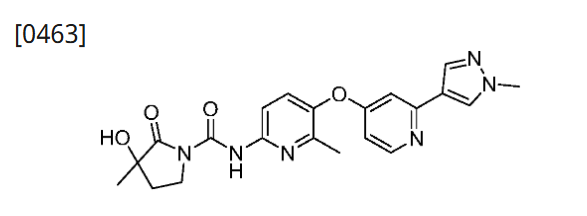
[0464]Palladium carbon (50 mg) was added to a solution of 3-(benzyloxy)-3-methyl-N-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)pyridin-4-yl)oxy)pyridin-2-yl)-2-carbonylpyrrolidine-1-carboxamide (80 mg, 0.15 mmol) in methanol (10 mL). The reaction was stirred at 50° C. for 2 hours in the presence of hydrogen. Filtered and concentrated. Plate chromatography (dichloromethane/methanol=18:1) gave 3-hydroxy-3-methyl-N-(6-methyl-5-((2-(1-methyl-1H-pyrazol-4-yl)pyridin-4-yl)oxy)pyridin-2-yl)-2-carbonylpyrrolidine-1-carboxamide (10 mg, yield 15%). MS m/z(ESI):423[M+1]
[0465]
1H NMR(400MHz,DMSO-d 6)δ10.93(s,1H),8.37(d,J=5.7Hz,1H),8.27(s,1H),8.01–7.85(m,2H),7.67(d,J=8.8Hz,1H),7.19(d,J=2.4Hz,1H),6.62(dd,J=5.7,2.4Hz,1H),5.89(s,1H),3.86(s,3H),3.83–3.76(m,1H),3.67–3.61(m,1H),2.29(s,3H),2.09–1.98(m,2H),1.34(s,3H)。
PATENTS
EP3643715
WO2018233527
US20200140431
US11180495 WO2018233527 US20200140431
References
- ^ Vaynrub A, Healey JH, Tap W, Vaynrub M (2022). “Pexidartinib in the Management of Advanced Tenosynovial Giant Cell Tumor: Focus on Patient Selection and Special Considerations”. OncoTargets and Therapy. 15: 53–66. doi:10.2147/OTT.S345878. PMC 8763255. PMID 35046667.
- ^ Merck Strengthens Oncology Portfolio Through Commercialization Agreement With Abbisko for Phase III Asset, Pimicotinib. (2023) https://www.merckgroup.com/en/news/abbisko-pimicotinib-agreement-04-12-2023.html
- ^ Merck KGaA buys into Abbisko’s late-stage joint tumor med for $70M upfront. Fierce Biotech. (2023) https://www.fiercebiotech.com/biotech/merck-kgaa-buys-abbiskos-late-stage-joint-tumor-med-70m-upfront
- ^ “Abbisko Therapeutics Announced the Entry into a Licensing Agreement for Pimicotinib (ABSK021) with Merck”. www.prnewswire.com (Press release). Retrieved 18 April 2024.
- ^ Study of Pimicotinib (ABSK021) for Tenosynovial Giant Cell Tumor (MANEUVER). U. S. National Institutes of Health, National Cancer Institute. https://classic.clinicaltrials.gov/ct2/show/NCT05804045
- ^ A Phase II Study Evaluating the Efficacy and Safety of ABSK021 (Pimicotinib)) in the Treatment of cGvHD Chronic Graft Versus Host Disease (cGvHD)U. S. National Institutes of Health, National Cancer Institute.https://classic.clinicaltrials.gov/ct2/show/NCT06186804
- ^ “FDA Grants Breakthrough Therapy Designation to Abbisko’s Pimicotinib”. Global genes. Retrieved 18 April 2024.
- ^ “Pimicotinib”. TGCT Support. Retrieved 18 April 2024.
- ^ “A Phase 3, Randomized, Double-blind, Placebo-Controlled, Multicenter Study of ABSK021 to Assess the Efficacy and Safety in Patients With Tenosynovial Giant Cell Tumor”. clinicaltrials. clinicaltrials.gov. 10 April 2024. Retrieved 18 April 2024.
External links
- “Pimicotinib”. NCI Drug Dictionary. National Cancer Institute.
- Clinical trial number NCT06186804 for “A Phase II Study Evaluating the Efficacy and Safety of ABSK021 (Pimicotinib)) in the Treatment of cGvHD Chronic Graft Versus Host Disease (cGvHD)” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Other names | ABSK021 |
| Routes of administration | Oral |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 2253123-16-7 |
| PubChem CID | 139549388 |
| ChemSpider | 128942304 |
| UNII | HV1XI8HST2 |
| KEGG | D12938 |
| Chemical and physical data | |
| Formula | C22H24N6O3 |
| Molar mass | 420.473 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
- [1]. Zhao BW, et al. N-(azaaryl)cyclolactam-1-carboxamide derivative, preparation method and application. World Intellectual Property Organization, WO2018214867 A1. 2018-11-29.[2]. Yang S, et al. Abstract LB-288: A highly selective small molecule CSF-1R inhibitor demonstrates strong immunomodulatory activity in syngeneic models. Cancer Research, 2018, 78(13_Supplement): LB-288-LB-288.[3]. Zhang N, et al. Abstract LB077: CSF-1R inhibition with Pimicotinib (ABSK021) enhanced anti-tumor efficacy of KRASG12C inhibitors in preclinical non-small cell lung cancer mouse models. Cancer Research, 2024, 84(7_Supplement): LB077-LB077.
//////////PIMICOTINIB, ABSK 021, Abbisko Therapeutics, HV1XI8HST2














