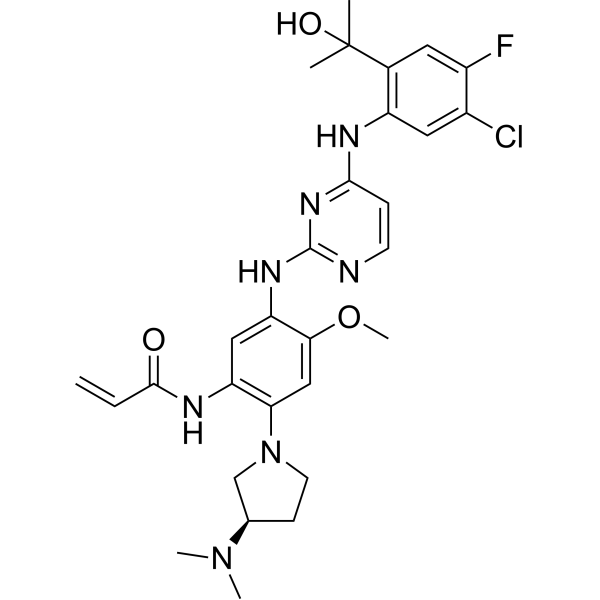
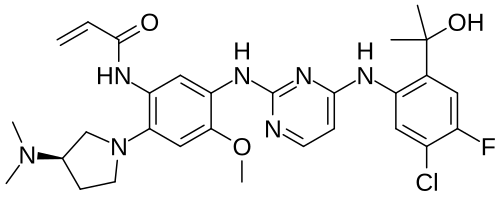
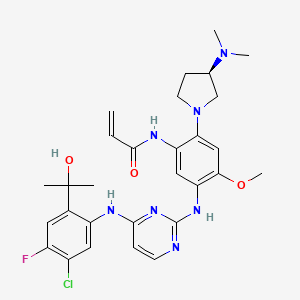
Sunvozertinib
CAS 2370013-12-8
DZD9008, 584.1 g/mol, C29H35ClFN7O3, A-1801, L1Q2K5JYO8
N-[5-[[4-[5-chloro-4-fluoro-2-(2-hydroxypropan-2-yl)anilino]pyrimidin-2-yl]amino]-2-[(3R)-3-(dimethylamino)pyrrolidin-1-yl]-4-methoxyphenyl]prop-2-enamide
FDA Zegfrovy, 7/2/2025
To treat locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations, as detected by an FDA-approved test, with disease progression on or after platinum-based chemotherapy |
Sunvozertinib (DZD9008) is a potent ErbBs (EGFR, Her2, especially mutant forms) and BTK inhibitor. Sunvozertinib shows IC50s of 20.4, 20.4, 1.1, 7.5, and 80.4 nM for EGFR exon 20 NPH insertion, EGFR exon 20 ASV insertion, EGFR L858R and T790M mutations, and Her2 Exon20 YVMA, and EGFR WT A431, respectively (patent WO2019149164A1, example 52).
Sunvozertinib, sold under the brand name 舒沃哲, among others is an anti-cancer medication used for the treatment of non-small-cell lung cancer.[2][3] It is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor.[2][4]
Sunvozertinib was approved for medical use in the United States in July 2025.[1]
Medical uses
In the US, sunvozertinib is indicated for the treatment of adults with locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.[1]
Side effects
The US FDA prescribing information for sunvozertinib includes warnings and precautions for interstitial lung disease/pneumonitis, gastrointestinal adverse reactions, dermatologic adverse reactions, ocular toxicity, and embryo-fetal toxicity.[1]
History
Sunvozertinib is being developed by Dizal Pharmaceutical.[5] In China, it was conditionally approved in 2023 for the treatment of NSCLC and full approval is contingent on results of phase 3 clinical trials.[6] In the United States, it has been designated by the Food and Drug Administration as a breakthrough therapy for patients with locally advanced or metastatic NSCLCs with an EGFR exon 20 insertion mutation.[7]
Efficacy was evaluated in WU-KONG1B (NCT03974022), a multinational, open-label, dose randomization trial.[1] Eligible participants had locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations with disease progression on or after platinum-based chemotherapy.[1] The primary efficacy population was in 85 participants who received sunvozertinib 200 mg orally once daily with food until disease progression or intolerable toxicity.[1]
The US Food and Drug Administration granted the application for sunvozertinib priority review and breakthrough therapy designations.[1]
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019149164&_cid=P10-MCWSEU-52423-1
[1286]
(R) -N- (5- (4- (5-chloro-4-fluoro-2- (2-hydroxypropan-2-yl) phenylamino) pyrimidin-2-ylamino) -2- (3- (dimethylamino) pyrrolidin-1-yl) -4-methoxyphenyl) acrylamide
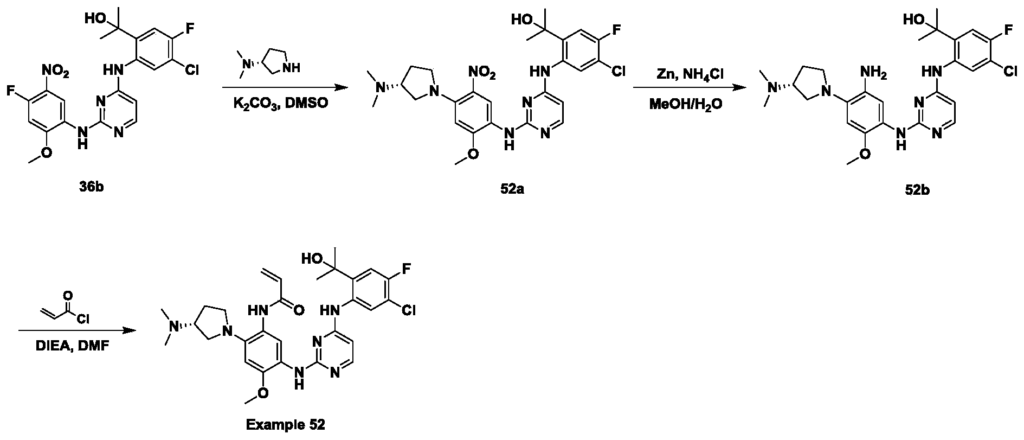
Procedure for the preparation of compound 52a:
[1289]
To a solution of compound 36b (200 mg, 0.429 mmol) and K 2CO 3(119 mg, 0.858 mmol) in DMSO (3 mL) was added (R) -N, N-dimethylpyrrolidin-3-amine (59 mg, 0.515 mmol) . The reaction mixture was stired at 22-30℃ for 4 h, then 50℃ for 1 h while color changed from brown to deep orange. The reaction mixture was added drop wise into H 2O (40 mL) under ice water bath. The precipitated solid was collected by filtration and washed with H 2O (15 mL × 3) , the filter cake was dissolved with CH 2Cl 2(20 mL) , dried over Na 2SO 4and concentrated in vacuum to give compound 52a (210 mg, 87.4%yield) as an orange solid.
[1290]
LCMS: R t=0.677 min in 5-95AB_220&254. lcm chromatography (MERCK RP18 2.5-2mm) , MS (ESI) m/z=559.9 [M+H] +.
[1291]
1H NMR (400MHz, Methanol-d 4) δ 8.42 (s, 1H) , 7.99 (d, J=7.6 Hz, 1H) , 7.96 (d, J=5.6 Hz, 1H) , 7.24 (d, J=10.8 Hz, 1H) , 6.50 (s, 1H) , 6.15 (d, J=5.6 Hz, 1H) , 3.96 (s, 3H) , 3.54 (dt, J=6.4, 10.4 Hz, 1H) , 3.36 (t, J=9.2 Hz, 1H) , 3.28 -3.24 (m, 1H) , 3.09 (dd, J=6.8, 10.0 Hz, 1H) , 2.93 -2.81 (m, 1H) , 2.33 (s, 6H) , 2.30 -2.25 (m, 1H) , 1.97 -1.82 (m, 1H) , 1.59 (d, J=3.6 Hz, 6H) .
[1292]
Procedure for the preparation of compound 52b:
[1293]
To a solution of compound 52a (210 mg, 0.375 mmol) in 5 mL MeOH/H 2O=5/1 (v/v) was added Zn (147 mg, 2.25 mmol) and NH 4Cl (120 mg, 2.25 mmol) . The resulting mixture was heated at 90℃ for 2 h while color changed from orange to brown. The reaction mixture was filtered, and the filtrate was concentrated in vacuum to give the crude residue, which was dissolved with CH 2Cl 2(20 mL) , washed with water (15 mL×3) , then dried over Na 2SO 4and concentrated in vacuum to give compound 52b (125 mg, 63%yield) as a brown solid.
[1294]
LCMS: R t=0.629 min in 5-95AB_220&254. lcm chromatography (MERCK RP18 2.5-2mm) , MS (ESI) m/z=530.1 [M+H] +.
[1295]
1H NMR (400MHz, CDCl 3) δ 8.85 (s, 1H) , 8.15 (d, J=7.2 Hz, 1H) , 8.03 (d, J=6.0 Hz, 1H) , 7.87 (s, 1H) , 7.43 (s, 1H) , 7.08 (d, J=10.4 Hz, 1H) , 6.66 (s, 1H) , 6.05 (d, J=5.6 Hz, 1H) , 3.81 (s, 3H) , 3.20 -3.11 (m, 2H) , 3.06 -2.97 (m, 2H) , 2.91 -2.83 (m, 1H) , 2.28 (s, 6H) , 2.17 -2.09 (m, 1H) , 1.92 -1.81 (m, 1H) , 1.65 (s, 6H) .
[1296]
Procedure for the preparation of Example 52:
[1297]
To a solution of compound 52b (125 mg, 0.236 mmol) and DIEA (46 mg, 0.354 mmol) in DMF (1.5 mL) was added acryloyl chloride (21 mg, 0.236 mmol) in ice water bath. The resulting mixture was stirred at 5-10℃ for 15 min. The reaction was quenched by H 2O (0.1 mL) and then filtered, the filtrate was purified by pre-HPLC directly (Column: Xtimate C18 150*25mm*5um; Condition: 35-65%B (A: 0.04%NH 3·H 2O+10mM NH 4HCO 3, B: CH 3CN) ; Flow Rate: 30 ml/min) and then lyophilized to give Example 52 (14.5 mg, 10.5%yield) as an off-white solid.
[1298]
LCMS: R t=2.028 min in 10-80CD_3min_220&254. lcm chromatography (XBrige Shield RP18 2.1*50mm, 5um) , MS (ESI) m/z=584.3 [M+H] +.
[1299]
1H NMR (400MHz, CDCl 3) δ 9.62 (s, 1H) , 9.43 (br s, 1H) , 8.59 (br s, 1H) , 8.08 (d, J=5.2 Hz, 1H) , 7.53 (d, J=6.8 Hz, 1H) , 7.47 (br s, 1H) , 7.14 (d, J=10.8 Hz, 1H) , 6.75 (s, 1H) , 6.41 -6.31 (m, 3H) , 5.77 (t, J=5.4 Hz, 1H) , 3.86 (s, 3H) , 3.15 -3.02 (m, 4H) , 2.97 -2.84 (m, 1H) , 2.30 (s, 6H) , 2.21 -2.14 (m, 1H) , 1.99 -1.94 (m, 1H) , 1.72 (s, 6H) .
PATENT
PAPER
https://www.mdpi.com/1420-3049/29/7/1448
Sunvozertinib, a novel TKI manufactured by Dizal Pharmaceuticals, represents an advancement arising from the need to overcome resistance mechanisms and thereby replaces previous generations of EGFR inhibitors. As marketed under the proprietary name DZD9008, this therapeutic entity embodies an innovative modality aimed at addressing NSCLC characterized by distinct mutations within the EGFR gene [24]. The pharmacological efficacy of sunvozertinib is predicated upon its specific capacity to inhibit EGFR with Exon 20 mutations, alongside targeting human epidermal growth factor receptor 2 (HER2) with Exon 20 insertions. This targeted inhibition is of significance due to the diminished responsiveness of cancer cells with these mutations to earlier generations of EGFR inhibitors. By competitively obstructing the ATP-binding site of these mutated tyrosine kinases, sunvozertinib effectively hinders the proliferative signaling pathways, exhibiting potent anti-tumor activity (ClinicalTrials.gov Identifier: NCT03974022). Preclinical models have demonstrated sunvozertinib’s capacity to inhibit tumor growth, especially in NSCLC models harboring Exon 20 insertions. Moreover, its substantiated ability to traverse the blood–brain barrier has provided favorable support for its prospective efficacy in managing central nervous system metastases. In clinical settings, sunvozertinib has shown promising efficacy, with ongoing trials further assessing its utility as a targeted intervention for individuals presenting specific EGFR mutations. The toxicity profile has been comparable to other EGFR inhibitors, with manageable side effects that do not significantly diminish its therapeutic value [25].
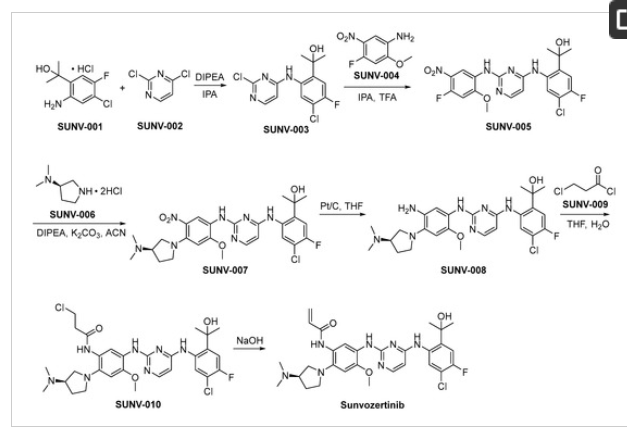
The preparation of sunvozertinib is shown in Scheme 1 [26]. SUNV-001 and SUNV-002 engage in nucleophilic substitution reactions under alkaline conditions, yielding the formation of SUNV-003 through a subsequent nucleophilic substitution process involving SUNV-004, ultimately leading to the generation of SUNV-005. SUNV-005 and SUNV-006 consecutively engage in nucleophilic substitution reactions, culminating in the formation of SUNV-007. The nitro moiety present in SUNV-007 undergoes a reduction process to yield an amino functional group, through the utilization of hydrogen gas as the reducing agent and platinum carbon as the catalytic mediator, ultimately affording the formation of SUNV-008. The amino moiety present in SUNV-008 and the acyl chloride functionality of SUNV-009 engage in a condensation reaction, leading to the formation of the amide compound SUNV-010. SUNV-010 is subjected to an elimination reaction in alkaline environments, leading to the formation of sunvozertinib.
References
- ^ Jump up to:a b c d e f g h “FDA grants accelerated approval to sunvozertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations”. U.S. Food and Drug Administration (FDA). 2 July 2025. Retrieved 7 July 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Jump up to:a b Wang M, Yang JC, Mitchell PL, Fang J, Camidge DR, Nian W, et al. (2022). “Sunvozertinib, a Selective EGFR Inhibitor for Previously Treated Non–Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations”. Cancer Discovery. 12 (7): 1676–1689. doi:10.1158/2159-8290.CD-21-1615. PMC 9262839. PMID 35404393.
- ^ Wang M, Fan Y, Sun M, Wang Y, Zhao Y, Jin B, et al. (2024). “Sunvozertinib for patients in China with platinum-pretreated locally advanced or metastatic non-small-cell lung cancer and EGFR exon 20 insertion mutation (WU-KONG6): Single-arm, open-label, multicentre, phase 2 trial”. The Lancet Respiratory Medicine. 12 (3): 217–224. doi:10.1016/S2213-2600(23)00379-X. PMID 38101437.
- ^ Hidetoshi Hayashi (2024). “Sunvozertinib: the next candidate of TKI for NSCLC”. The Lancet Respiratory Medicine. 12 (3): 185–186. doi:10.1016/S2213-2600(23)00419-8. PMID 38101435.
- ^ “ASH: With high tumor response, AstraZeneca spinout Dizal explores FDA path and US partner for PTCL drug”. Fierce Biotech. 11 December 2023.
- ^ Dhillon S (2023). “Sunvozertinib: First Approval”. Drugs. 83 (17): 1629–1634. doi:10.1007/s40265-023-01959-5. PMID 37962831.
- ^ “FDA Grants Breakthrough Therapy Designation to Sunvozertinib in EGFR Exon20+ NSCLC”. targetedonc.com. 9 April 2024.
External links
- Clinical trial number NCT03974022 for “Assessing an Oral EGFR Inhibitor, Sunvozertinib in Patients Who Have Advanced Non-small Cell Lung Cancer with EGFR or HER2 Mutation (WU-KONG1)” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Trade names | 舒沃哲, Zegfrovy |
| Routes of administration | Oral |
| ATC code | None |
| Legal status | |
| Legal status | US: ℞-only[1]Rx in China |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 2370013-12-8 |
| PubChem CID | 139377809 |
| DrugBank | DB18925 |
| UNII | L1Q2K5JYO8 |
| Chemical and physical data | |
| Formula | C29H35ClFN7O3 |
| Molar mass | 584.09 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
//////////Sunvozertinib, DZD9008, DZD 9008, FDA 2025, APPROVALS 2025, A-1801, A 1801, L1Q2K5JYO8














