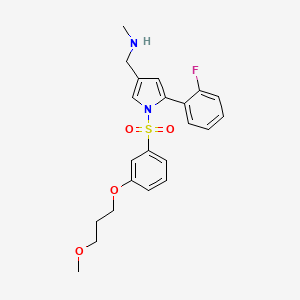
- P-CAB agent 2
- keverprazan
- 1978371-23-1
- Keprason
- SOC12UY3ZP
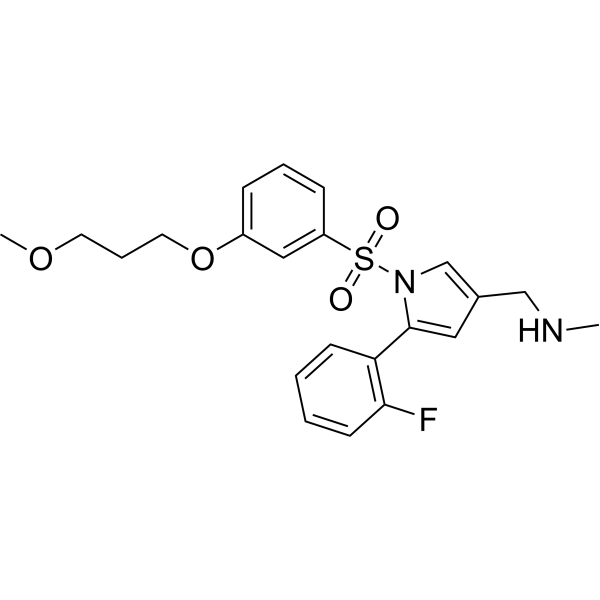
432.5 g/mol
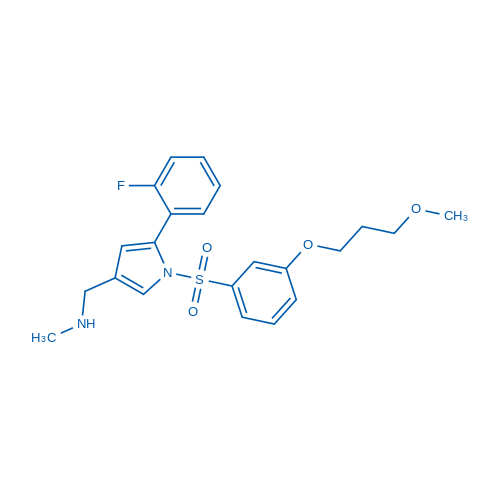
1-[5-(2-fluorophenyl)-1-[3-(3-methoxypropoxy)phenyl]sulfonylpyrrol-3-yl]-N-methylmethanamine
C22H25FN2O4S
- 1H-Pyrrole-3-methanamine, 5-(2-fluorophenyl)-1-[[3-(3-methoxypropoxy)phenyl]sulfonyl]-N-methyl-
- 5-(2-Fluorophenyl)-1-[[3-(3-methoxypropoxy)phenyl]sulfonyl]-N-methyl-1H-pyrrole-3-methanamine
- Keverprazan Hydrochloride: First ApprovalPublication Name: DrugsPublication Date: 2023-04-19PMID: 37074491DOI: 10.1007/s40265-023-01865-w
- Efficacy of keverprazan for duodenal ulcer: A phase II randomized, double‐blind, parallel‐controlled trialPublication Name: Journal of Gastroenterology and HepatologyPublication Date: 2022-09-16PMID: 36068945DOI: 10.1111/jgh.16000
- The efficacy and safety of keverprazan, a novel potassium‐competitive acid blocker, in treating erosive oesophagitis: a phase <scp>III</scp>, randomised, double‐blind multicentre studyPublication Name: Alimentary Pharmacology & TherapeuticsPublication Date: 2022-05-03PMID: 35505467DOI: 10.1111/apt.16959
- Potassium-competitive acid blockers – are they the next generation of proton pump inhibitors?Publication Name: World Journal of Gastrointestinal Pharmacology and TherapeuticsPublication Date: 2018-12-13PMCID: PMC6305499PMID: 30595950DOI: 10.4292/wjgpt.v9.i7.63
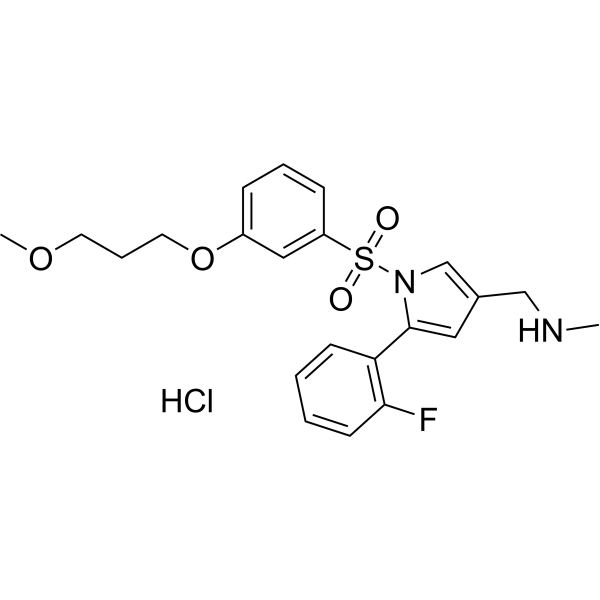
CAS 2209911-80-6
| Molecular Weight | 468.97 |
|---|---|
| Formula | C22H26ClFN2O4S |
Jiangsu Carephar Pharmaceuticals, is
a potassium ion competitive acidblocker (P-CAB) that was approved inFebruary2023inChina for thetreatment of refluxesophagitis or duodenal ulcer inadults
P-CAB agent 2 hydrochloride is a potent and orally active potassium-competitive acid blocker and a gastric acid secretion inhibitor. P-CAB agent 2 hydrochloride inhibits H+/K+-ATPase activity with an IC50 value of <100 nM. P-CAB agent 2 hydrochloride inhibits the hERG potassium channel with an IC50 value of 18.69 M. P-CAB agent 2 hydrochloride shows no acute toxicity and inhibits histamine (HY-B1204)-induced gastric acid secretion.
P-CAB agent 2 is a potent and orally active potassium-competitive acid blocker and a gastric acid secretion inhibitor. P-CAB agent 2 inhibits H+/K+-ATPase activity with an IC50 value of <100 nM. P-CAB agent 2 inhibits the hERG potassium channel with an IC50 value of 18.69 M. P-CAB agent 2 shows no acute toxicity and inhibits histamine (HY-B1204)-induced gastric acid secretion[1].
REF
https://pubs.acs.org/doi/10.1021/acs.jmedchem.4c02079?ref=PDF
KeverprazanHydrochloride. Keverprazan hydrochloride(4),developedby Jiangsu CarepharPharmaceuticals,is
apotassiumioncompetitiveacidblocker (P-CAB) thatwas approvedinFebruary2023inChina forthetreatmentofrefluxesophagitisorduodenal ulcer inadults.36Those illnesses arecaused by gastric acid entering the esophagus, leading toregurgitation and heartburn.37 Current treatments mainly
employ acid-suppressing therapies such as proton pumpinhibitors (PPIs, e.g., lansoprazole); however, PPIs do notacidicenvironments.38Toaddress those issues,P-CABshavebeen developed as a new class of acid suppressants.39
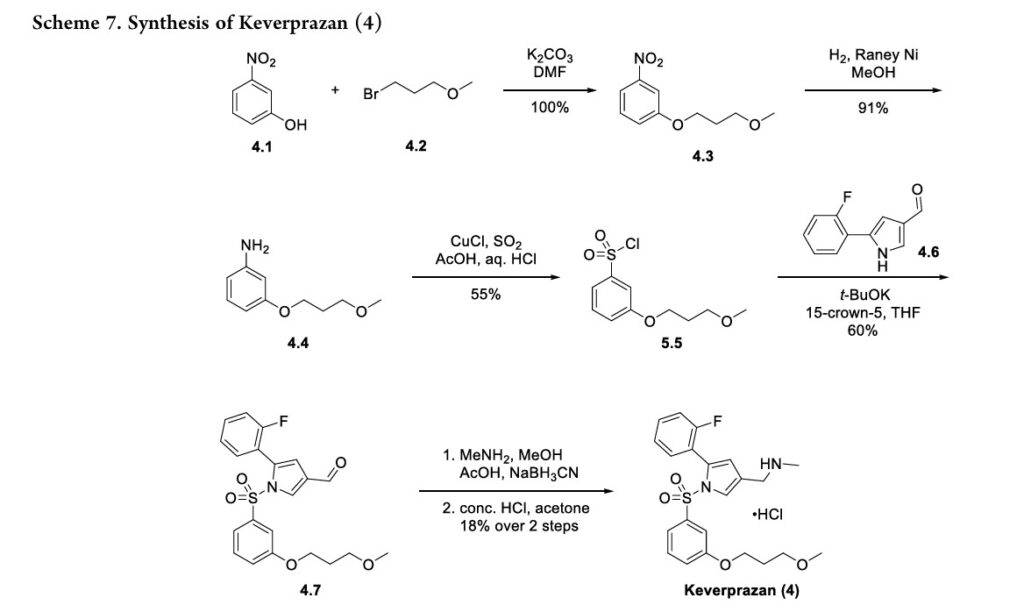
Keverprazan, a novel P-CAB, reversibly binds to gastricH+,K+-ATPase in competition against K+ ions, therebyinhibitingtheenzyme.40Itprovidesstableandsustainedgastricacidinhibitioneffectat20mgdose,whilebeingsafeandwelltoleratedupto60mginasingle-ascendingdosestudy.41Theoriginal synthesisof keverprazanhydrochloridedevelopedbyQinetal. isillustratedinScheme7.42TheroutebeganwithSN2alkylationof3-nitrophenol4.1withalkylbromide4.2
toformphenylether4.3.Thenitrogroupwasreducedtoaniline 4.4underRaney-Ni-mediatedhydrogenation.Aniline4.4wasconverted to sulfonyl chloride 4.5 via copper-mediated
sulfonylationin55%yield.Thesulfonyl chloridewascoupledwithpyrrolederivative4.6toformsulfonamide4.7in60%yield.
Finally, reductiveaminationofthealdehydewithmethylamine and sodium cyanoborohydride, followed by HCl salt formation,43furnishedkeverprazanhydrochloride(4)in18%yield.
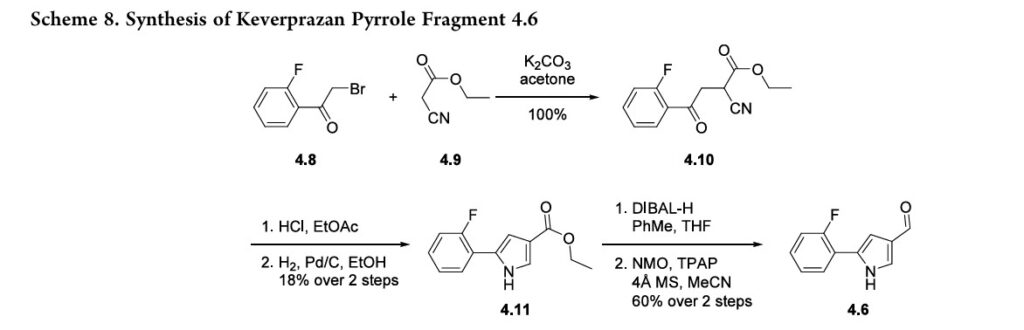
The synthesis of pyrrole fragment 4.6was reported byArikawaetal.andillustratedinScheme8.39SN2displacementofα-bromoketone 4.8 with ethyl cyanoacetate 4.9 affordedintermediate 4.10. Treatment of 4.10 with anhydrous HCl in EtOAc followed by hydrogenation effected cyclization to form pyrazole 4.11. The ester was reduced to the corresponding
alcohol with DIBAL-H,andthealcoholoxidized to aldehyde4.6 with NMO and TPAP.
(36) Kang, C.Keverprazan Hydrochloride: first approval. Drugs 2023,
83, 639−643.
(37) Chen, S.; Liu, D.; Chen, H.; Liao, A.; Li, F.; Liu, C.; Li, X.; Li, S.;
Zhang, Y.; Wang, Y.; et al. The efficacy and safety of keverprazan, a
novel potassium-competitive acid blocker, in treating erosive
oesophagitis: a phase III, randomised, double-blind multicentre
study. Aliment. Pharmacol. Ther. 2022, 55, 1524−1533.
(38)Tan,N.-d.; Liu, X.-w.; Liu, C.-x.; Li, S.-b.; Chen, H.-h.; Li, X.; Wu,
H.; Liao, A.-J.; Zhen, Y.-b.; Shen, P.-z.; et al. Efficacy of keverprazan for
duodenal ulcer: a phase II randomized, double-blind, parallel
controlled trial. J. Gastroenterol. Hepatol. 2022, 37, 2060−2066.
(39) Arikawa, Y.; Nishida, H.; Kurasawa, O.; Hasuoka, A.; Hirase, K.;
Inatomi, N.; Hori, Y.; Matsukawa, J.; Imanishi, A.; Kondo, M.; et al.
Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1
(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fuma
rate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J.
Med. Chem. 2012, 55, 4446−4456.
(40) Parsons, M. E.; Keeling, D. J. Novel approaches to the
pharmacological blockade of gastric acid secretion. Expert Opin. Invest.
Drugs 2005, 14, 411−421.
(41) Zhou,S.;Xie, L.; Zhou, C.; Zhao, Y.; Wang,L.;Ding,S.;Chen, J.;
Zhu, B.; Su, M.; Shao, F. Keverprazan, a novel potassium-competitive
acid blocker: single ascending dose safety, tolerability, pharmacoki
netics, pharmacodynamics and food effect in healthy subjects. Eur. J.
Pharm. Sci. 2023, 190, No. 106578.
(42) Qin, Y.; Su, M.; Jin, Q.; Chen, T.; Jiang, J. Pyrrole sulfonyl
derivative, and preparation method and medical use thereof. EP
3248963 B1, 2019.
SYN
WO2016119505
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016119505&_cid=P12-MD5D4X-02931-1
[0042]Preparation of 1-(5-(2-fluorophenyl)-1-((3-(3-methoxypropoxy)phenyl)sulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine (EXP 1)
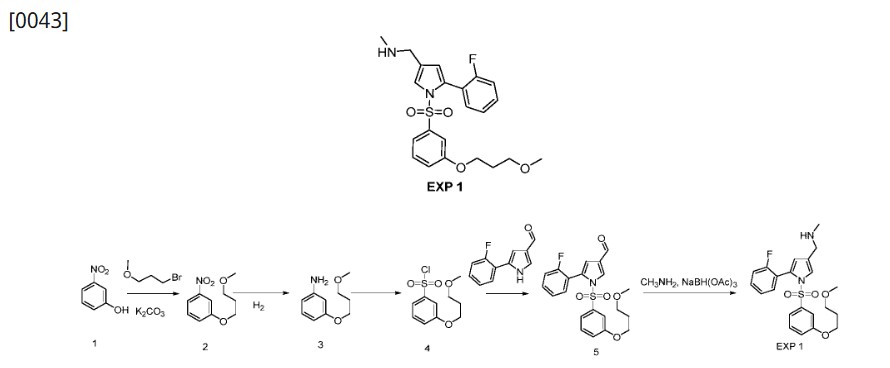
1) Preparation of 1-(3-methoxypropoxy)-nitrobenzene (Compound 2)
[0045]3-Nitrophenol (Compound 1, 1.0 g, 7.19 mmol), potassium carbonate (2.9 g, 21.6 mmol) and 1-bromo-3-methoxypropane (1.65 g, 10.79) were dissolved in anhydrous DMF (20 mL), stirred at 90 °C overnight, added with water (50 mL), and extracted with ethyl acetate (50 mL x 3). The organic phases were combined, dried, and concentrated to give a yellow solid 1-(3-methoxypropoxy)-nitrobenzene (Compound 2, 1.5 g, yield 100%).
[0046]2) Preparation of 1-(3-methoxypropoxy)-aniline (Compound 3)
[0047]1-(3-methoxypropoxy)-nitrobenzene (Compound 2, 1.0 g, 4.74 mmol) and Raney Ni (100 mg) were dissolved in anhydrous methanol (20 mL) and stirred overnight at room temperature under a hydrogen atmosphere. The mixture was filtered and the filtrate was dried to obtain solid 1-(3-methoxypropoxy)-aniline (Compound 3, 0.80 g, 91% yield).
[0048]3) Preparation of 1-(3-methoxypropoxy)-benzenesulfonyl chloride (Compound 4)
[0049]At 0°C, sodium nitrite (571 mg, 8.29 mmol) was added to acetic acid (10 mL) and aqueous hydrochloric acid solution (2N, 10 mL) of 1-(3-methoxypropoxy)-aniline (compound 3, 1.0 g, 5.52 mmol) in batches. After the addition was completed, the mixture was stirred at 0°C for 25 min to obtain solution I. At 0°C, cuprous chloride (190 mg, 1.1 mmol) was added to an acetic acid solution of sulfur dioxide (10 mL, 2N) to obtain solution II. At 0°C, solution I was added dropwise to solution II, and after the addition was completed, the mixture was naturally warmed to room temperature and stirred for 3 hours. The mixture was extracted with ethyl acetate (150 mL x 3), the organic phases were combined, dried, concentrated, and column chromatography (petroleum ether: ethyl acetate = 20:1) was performed to obtain 1-(3-methoxypropoxy)-benzenesulfonyl chloride (compound 4, 800 mg, yield 55%) as a yellow oil.
[0050]4) Preparation of 5-(2-fluorophenyl)-1-((3-(3-methoxypropoxy)phenyl)sulfonyl)-1H-pyrrole-3-carbaldehyde (Compound 5)
[0051]At 0°C, t-BuOK (233 mg, 2.08 mmol) was added to a solution of 5-(2-fluorophenyl)-1H-pyrrole-3-carboxaldehyde (200 mg, 1.04 mmol) in anhydrous THF (5 mL), and the mixture was stirred at 0°C for 30 min. After the reaction was completed, 15-crown-5 (542 mg, 2.08 mmol) and 1-(3-methoxypropoxy)-benzenesulfonyl chloride (compound 4, 412 mg, 2.08 mmol) were added respectively. After the addition was completed, the mixture was naturally heated to room temperature and stirred for 90 min. After the reaction was completed, the mixture was quenched with ice water (50 g) and extracted with ethyl acetate (50 mL x 3). The organic phases were combined, dried, concentrated, and purified by column chromatography (petroleum ether:ethyl acetate=8:1) to give a yellow solid 5-(2-fluorobenzene)-1-((3-(3-methoxypropoxy)phenyl)sulfonyl)-1H-pyrrole-3-carbaldehyde (260 mg, yield 60%).
[0052]5) Preparation of 1-(5-(2-fluorophenyl)-1-((3-(3-methoxypropoxy)phenyl)sulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine (EXP 1)
[0053]5-(2-Fluorobenzene)-1-((3-(3-methoxypropoxy)phenyl)sulfonyl)-1H-pyrrole-3-carbaldehyde (Compound 5, 500 mg, 1.19 mmol), acetic acid (144 mg, 2.39 mmol), and methylamine alcohol solution (1 mL) were dissolved in 3 mL of anhydrous methanol and stirred at room temperature for 4 h. NaBH
3 CN (212 mg, 3.59 mmol) was added. The mixture was stirred for 60 min. Ice water (30 g) was added to quench the reaction and the mixture was extracted with ethyl acetate (50 mL x 3). The organic phases were combined, dried, concentrated, and subjected to column chromatography to obtain 1-(5-(2-fluorobenzene)-1-((3-(3-methoxypropoxy)phenyl)sulfonyl)-1H-pyrrole-3-yl)-N-methylmethanamine (EXP 1, 100 mg, 20%) as a white solid.
[0054]
HPLC:99.4%;MS(ESI)m/z:[M+H] +=433.0; 1H-NMR(400MHz,DMSO-d6)δ:8.72(s,1H),7.78(d,1H),7.46-7.55(m,2H),7.21-7.32(m,3H),6.85-7.11(m,2H),6.83-6.85(m,1H),6.44(d,1H),3.95-4.02(m,4H),3.47(t,2H),3.32(s,3H),2.52(m,3H),1.94(t,2H)ppm。
[References]
[1] YinLin Qin, et al. Pyrrole sulfonyl derivative, and preparation method and medical use thereof. WO2016119505A1.
///////////KEVERPRAZAN, Keverprazan, CHINA 2023, APPROVAL 2023, Jiangsu Carephar Pharmaceuticals, ULCER



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com














