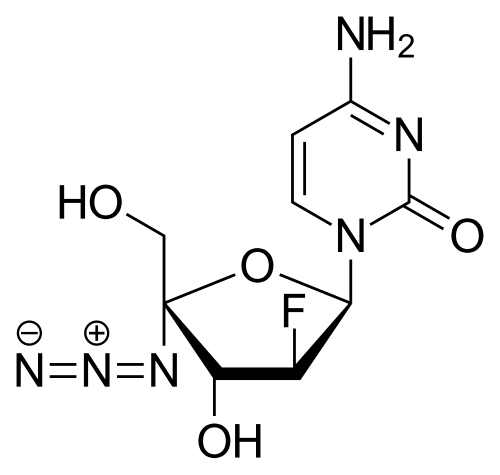
CAS
WeightAverage: 286.223
Monoisotopic: 286.082581021
Chemical FormulaC9H11FN6O4
- FNC
- HY-19314
- RO 0622
- RO-0622
- SB17040
IJ2XP0ID0K- DTXSID901027757
4-amino-1-[(2R,3S,4R,5R)-5-azido-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2-dihydropyrimidin-2-one
- 2′-Deoxy-2′-beta-fluoro-4′-azidocytidine
- 2(1H)-Pyrimidinone, 4-amino-1-(4-c-azido-2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-
- 4-amino-1-((2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-5-((imino-l,5-azanylidene)amino)tetrahydrofuran-2-yl)pyrimidin-2-one
- 4-amino-1-((2R,3S,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-5-((imino-l,5-azanylidene)amino)tetrahydrofuran-2-yl)pyrimidin-2-one
- 4-Amino-1-(4-c-azido-2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-2(1H)-pyrimidinone
- 4′-C-azido-2′-deoxy-2′-fluoro-beta-D-arabinocytidine
- 4-amino-1-[(2R,3S,4R,5R)-5-azido-3-fluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one
- AZVUDINE [WHO-DD]
- 4-amino-1-((2R,3S,4R,5R)-5-azido-3-fluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one
- 4-AMINO-1-(4-C-AZIDO-2-DEOXY-2-FLUORO-.BETA.-D-ARABINOFURANOSYL)-2(1H)-PYRIMIDINONE
- 2(1H)-PYRIMIDINONE, 4-AMINO-1-(4-C-AZIDO-2-DEOXY-2-FLUORO-.BETA.-D-ARABINOFURANOSYL)-
- 4′-C-Azido-2′-deoxy-2′-fluoro-b-D-arabinocytidine
Azvudine is an antiviral drug which acts as a reverse transcriptase inhibitor.[3] It was discovered for the treatment of hepatitis C[4] and has since been investigated for use against other viral diseases such as AIDS and COVID-19,[2][5] for which it was granted conditional approval in China.[6][7]
Azvudine was first discovered in 2007.[8] It costs 350 Chinese yuan per 7 days for COVID, as of November 2022.[9]
Azvudine is under investigation in clinical trial NCT04668235 (Study on Safety and Clinical Efficacy of AZVUDINE in COVID-19 Patients (Sars-cov-2 Infected)).
Azvudine (RO-0622) is a potent nucleoside reverse transcriptase inhibitor (NRTI), with antiviral activity on HIV, HBV and HCV. Azvudine exerts highly potent inhibition on HIV-1 (EC50s ranging from 0.03 to 6.92 nM) and HIV-2 (EC50s ranging from 0.018 to 0.025 nM). Azvudine inhibits NRTI-resistant viral strains. Azvudine is a click chemistry reagent, it contains an Azide group and can undergo copper-catalyzed azide-alkyne cycloaddition reaction (CuAAc) with molecules containing Alkyne groups. It can also undergo strain-promoted alkyne-azide cycloaddition (SPAAC) reactions with molecules containing DBCO or BCN groups.
SYN
https://patents.google.com/patent/CN111892636A/en
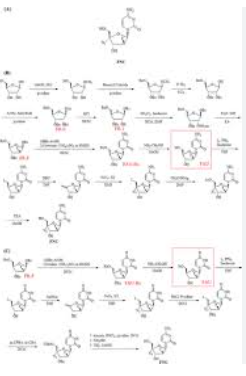
According to the inventor’s research and understanding, at present, the synthesis of azvudine mainly includes the following methods according to different raw materials:
1. It is prepared by using a ribonucleotide as a raw material. The method requires a total of 15 steps of reaction to obtain the target product. The inventor’s research found that DAST is used as a fluorination reagent in the fluorination process of this method, which has large steric hindrance and is difficult to fluoride, and the route process is complicated and the route is long. Low cost, high cost, not suitable for industrial production.
2. It is prepared by using 1,3,5-O-tribenzoyl-2-deoxy-2-fluoro-D-arabinofuranoside as raw material. The inventors have found that the preparation raw materials are not easy to obtain, and the route involves uridine to In the conversion reaction of cytidine, the reaction route is further extended, thus limiting the further application of this method.
3. Using ribonucleotides as raw materials to synthesize, the inventors found that this method requires 12 steps to synthesize the target product, and DAST is also used as a fluorination reagent in the fluorination process, which has large steric hindrance and low fluorination efficiency.
4. It is prepared by using uracil nucleotide as raw material. The inventors have found that the raw material cost of this method is relatively high, it involves the conversion process of uridine to cytidine, and the reaction yield is not high.
To sum up, the inventors have found that the currently known processes for preparing azvudine have the following disadvantages: synthesizing uracil nucleotides with ribonucleotides as raw materials, and then converting uracil nucleotides into target products, synthesizing uracil nucleotides. The process routes all exceed 12 steps, and the fluorination reaction uses DAST (diethylaminosulfur trifluoride) reagent, which increases the difficulty of the reaction due to steric hindrance, reduces the yield, and brings difficulties to industrial production.
Example 1


Accurately weigh 4.86 g of cytidine (compound 1), dissolve it in 20 mL of ethanol, then add 4.52 g of benzoic anhydride, raise the temperature to 60° C., and stir overnight. After the reaction was completed, the solvent was removed under reduced pressure, and 100 mL of deionized water was added to wash and filter to obtain compound 2 (96.5% yield). The structural characterization is shown in Figure 1 .
Example 2


Weigh 3.5 g of compound 2, dissolve in 25 mL of pyridine, ice-water bath at 0°C, add 3.0 mL of TIPDS under nitrogen protection, react for 1 h, decompose the reaction mixture with water, remove pyridine under reduced pressure, extract with chloroform, and wash with saturated aqueous sodium bicarbonate solution , and dried over anhydrous sodium sulfate to obtain compound 3 (83.6% yield). The structural characterization is shown in Figure 2.
Example 3


Weigh 5.9 g of compound 3, dissolve it in 100 mL of tetrahydrofuran, add 2.82 g of trifluoromethanesulfonic anhydride (Tf 2 O), and react at room temperature for 2 h under nitrogen protection. Then, the reaction temperature was lowered to -20°C, 2.46 g of tetrabutylammonium fluoride (Bu 4 NF) was added, and the reaction was continued for 10 h. After the reaction was completed, tetrahydrofuran was removed under reduced pressure, extracted with chloroform, washed with saturated aqueous sodium bicarbonate solution, and dried over anhydrous sodium sulfate to obtain compound 4 (86.8% yield). The structural characterization is shown in Figure 3.
Example 4


5.9 g of compound 3 was weighed, dissolved in 100 mL of dichloromethane and 10 mL of anhydrous pyridine, cooled to -50°C under nitrogen protection, added with 1.61 g of DAST, and reacted for 12 h. After the reaction was completed, the solvent was removed under reduced pressure, extracted with chloroform, washed with saturated aqueous sodium bicarbonate solution, and dried over anhydrous sodium sulfate to obtain compound 4 (76.0% yield).
Example 5


Weigh 3.5g of compound 4, add 100mL of tetrahydrofuran, add 0.5g of imidazole, 0.5g of triphenylphosphine, slowly add 3.75g of tetrahydrofuran solution containing 10wt% iodine, stir at room temperature for 5h, the reaction is complete, remove the solvent under reduced pressure, Compound 5 was prepared (84% yield) and the structural characterization is shown in Figure 4 .
Example 6


Weigh 4.59g of compound 5, dissolve it in 100mL of methanol, add 0.5g of DBU, control the temperature to 60°C, react for 12h, cool to room temperature, add saturated aqueous sodium chloride solution, adjust the acidic pH=3 with 1M hydrochloric acid, extract with ethyl acetate, It was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain compound 6 (77.4% yield). The structural characterization is shown in Figure 5 .
Example 7


Weigh 3.3 g of compound 6, add 50 mL of DMF solution dissolved with 0.6 g of sodium azide, add 50 mL of DMF solution dissolved with 0.6 g of ICl, control the temperature to 0 ° C, and react for 12 h. After the reaction is completed, add sodium bisulfite until The color of iodine disappears completely. The solvent was removed under reduced pressure to obtain compound 7 (77% yield). The structural characterization is shown in FIG. 6 .
Example 8


Weigh 2.5 g of compound 7, dissolve it in 50 mL of DMF, add 0.65 g of benzoic acid, add 0.5 g of silver acetate, and stir at room temperature for 12 h. After the reaction was completed, the mixture was filtered, and the solvent was removed under reduced pressure to obtain compound 8 (71.2% yield). The structural characterization is shown in FIG. 7 .
Example 9


Weigh 4.82 g of compound 8, add 100 mL of methanol, 10 mL of deionized water, 3 mL of triethylamine, stir at room temperature for 5 h, and remove the solvent under reduced pressure to obtain compound 9 (88.7% yield). The structural characterization is shown in Figure 8.
SYN
https://pubs.acs.org/doi/10.1021/acs.oprd.4c00166
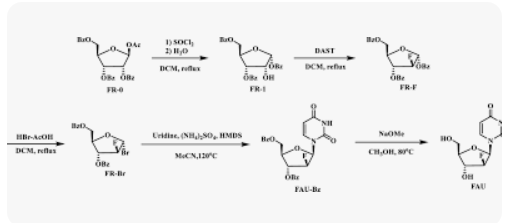
Azvudine was approved for the treatment of adult HIV-1 infection in China in 2021, and it was approved for conditional marketing for the treatment of SARS-CoV-2 in China in 2022. In this work, we describe a fully continuous flow synthesis of 2′-deoxy-2′-fluoroarabinoside, a key intermediate for azvudine. The process was accomplished via six chemical transformations, including chlorination, hydrolysis, fluorination, bromination, condensation, and deprotection in six sequential continuous flow devices. Under the optimized process conditions, the total yield was 32.3% with a total residence time of 156 min. Compared with batch conditions, the total yield was doubled, the total reaction time was shortened 16 times, and the E factor was reduced 1.63 times.
1,3,5-Tri-O-benzoyl-D-ribofuranose (FR-1)
To a stirred solution of O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose (FR-0,
1.008 g, 2.0 mmol) in anhydrous dichloromethane (DCM, 10 mL) was added
dropwise thionyl chloride (0.713 g,6 mmol) at 0 oC, and the resulting mixture
allowed to stir at room temperature for 14 h. The solution was evaporated,
dissolved with toluene (5 mL× 3), and then evaporated. To the residue was
added DCM (10 mL) and water (5 mL), and and continued stirring at room
temperature for 2 h. The solution was then washed with saturated aqueous
NaHCO3 (15 mL) and dried over Na2SO4, filtered and evaporated. DCM (2 mL)
and n-Hexane (20 mL) was added to the residue and the mixture was stirred at
room. A white solid was collected by filtration to give FR-1 (yield: 47.8%).
2-Deoxy-2-fluoro-1,3,5-tri-O-benzoyl-D-ribofuranose (FR-F)
To a stirred solution of FR-1 (0.924 g, 2.0 mmol) in anhydrous DCM (10 mL) was
added dropwise DAST (0.976 g, 6.0 mmol) for 30 min, and the resulting mixture
allowed to stir at 40 °C (16 h). The reaction was cooled and quenched with
saturated aqueous NaHCO3 (15 mL). The solution was extracted with DCM and
water, and then washed with saturated aqueous NaHCO3 (20 mL). This was then
dried over Na2SO4, filtered and evaporated. The crude product was purified by
silica gel column chromatography (ethyl acetate: petroleum ether =1:10) to
obtain a white solid (yield: 41.2%).
α-D-Arabinofuranosyl bromide, 2-deoxy-2-fluoro-, 3,5-dibenzoate (FR-Br)
To a stirred solution of FR-F (0.928 g, 2.0 mmol) in anhydrous DCM (10 mL) was
added dropwise 33.3% HBr-HOAc (1.47 g, 6.0 mmol) at 0 oC, and the resulting
mixture were allowed to stir at room temperature for 7 h. The reaction was
quenched with saturated aqueous NaHCO3 (10 mL), dried over Na2SO4, filtered
and evaporated to yield the product (FR-Br) as a brown oil (yield: 99.8%).
1-(2-deoxy-2-fluoro-3,5-di-O-benzoyl-β-D-arabino-furanosyl)uracil (FAU-Bz)
A mixture of uracil (0.336 g, 3 mmol) and (NH4)2SO4 (10 mg, 0.075 mmol) in
hexamethyldisilazane (HMDS) (6 mL) was refluxed under nitrogen for 5 h. To
the silylated uracil solution was added a solution of FR-Br in dry acetonitrile (10
mL) and the mixture was refluxed under nitrogen for 5 h. The solution was
evaporated, extracted with DCM (20 mL) and washed with saturated aqueous
NaHCO3 (15 mL). This was then dried over Na2SO4, filtered and evaporated.
Ethyl acetate (10 mL) and petroleum ether (50 mL) was added to the residue
and the mixture was stirred at room temperature. A light yellow solid was
collected by filtration to give FAU-Bz (yield: 83.2%).
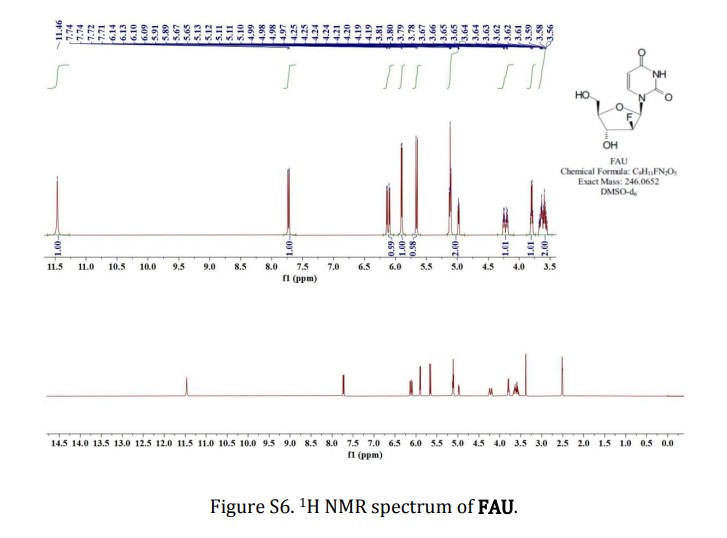
2′-deoxy-2′-fluoro- arabinoside (FAU)
To a solution of FUA-Bz (0.908 g, 2.0 mmol) in anhydrous methanol (MeOH, 10
mL) was added NH3-MeOH (5 mL), stirred at room temperature for 15 h and
evaporated to dryness under reduced pressure. White solid
SYN
: J. Med. Chem. 2024, 67, 4376−4418
Azvudine (1). Azvudine (1) is an antiviral manufactured by China-based Genuine Biotech. It was
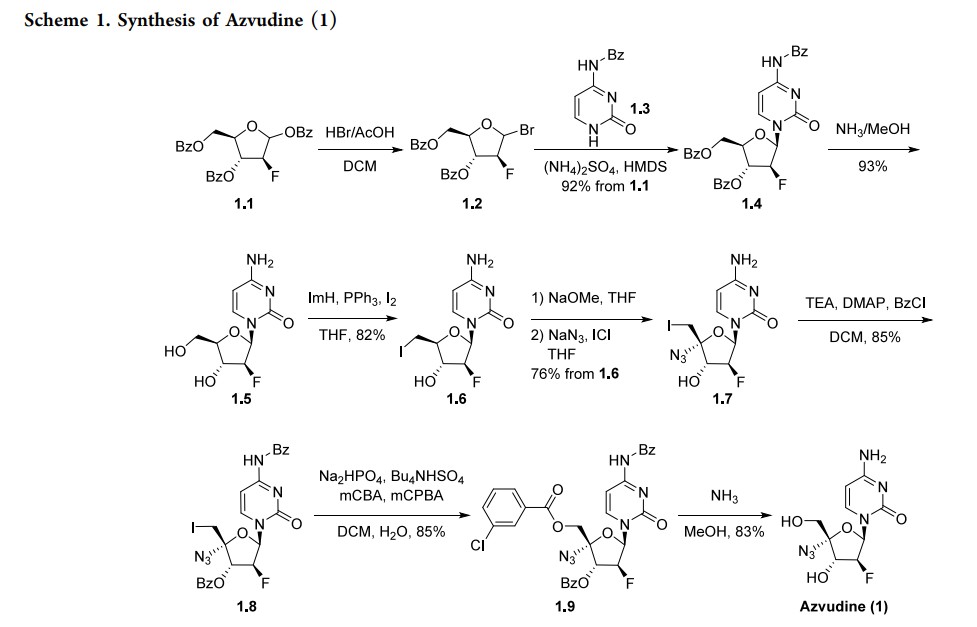
approved in China in 2021 as a first-in-class treatment for human immunodeficiency virus (HIV). It has a dual function, acting as a reverse transcriptase inhibitor and targeting the viral infectivity factor/apolipoprotein B mRNA-editing enzyme, catalytic subunit 3G (Vif/A3G) protein−protein interaction.6 Azvudine has activity against both wild-type and drug-resistant strains of HIV due to the presence of a 3′-hydroxy group and substitution in the 4′-position of the ribose core.7 Due to its known antiviral activity, azvudine was repurposed as a treatment for COVID-19 and approved for this indication inChina in 2022. It acts as an RNA-dependent RNA polymerase (RdRp) inhibitor, the same mechanism as the previously approved molnupiravir and remdesivir. In addition to its antiviral activity, concentration of the drug in the thymus has suggested immune-targeting activity; this dual function is unique among RdRp inhibitors.8 Several syntheses of azvudine have been reported in the scientific and patent literature. Scheme 1 highlights a 100 g scale synthesis from a patent filed by Shandong University.9 Other syntheses are similar, containing the furanose functional group manipulations in 1.5−1.8, though these routes differ in choice of nucleobase and protecting group strategy, and were reported on a smaller scale.10−12 The synthesis began from benzoyl-protected fluoro-furanose 1.1. Bromination with HBr in acetic acid followed by displacement of the bromide 1.2 with protected cytosine 1.3 yielded intermediate 1.4. Deprotection of the benzoyl groups with ammonia in MeOH formed diol 1.5, and a Mitsunobu reaction converted the
primary alcohol to alkyl iodide 1.6. Elimination of the iodide with sodium methoxide followed by addition of sodium azide and iodine monochloride across the resulting alkene produced substitution in the 4′-position of the ribose core.7 Due to its known antiviral activity, azvudine was repurposed as a treatment for COVID-19 and approved for this indication in China in 2022. It acts as an RNA-dependent RNA polymerase (RdRp) inhibitor, the same mechanism as the previously approved molnupiravir and remdesivir. In addition to its antiviral activity, concentration of the drug in the thymus has suggested immune-targeting activity; this dual function is unique among RdRp inhibitors.8 Several syntheses of azvudine have been reported in the scientific and patent literature. Scheme 1 highlights a 100 g scale synthesis from a patent filed by Shandong University.9 Other syntheses are similar, containing the furanose functional group manipulations in 1.5−1.8, though these routes differ in choice of nucleobase and protecting group strategy, and were reported on a smaller scale.10−12 The synthesis began from
benzoyl-protected fluoro-furanose 1.1. Bromination with HBr in acetic acid followed by displacement of the bromide 1.2 with protected cytosine 1.3 yielded intermediate 1.4. Deprotection of the benzoyl groups with ammonia in MeOH formed diol 1.5, and a Mitsunobu reaction converted the primary alcohol to alkyl iodide 1.6. Elimination of the iodide with sodium methoxide followed by addition of sodium azide
and iodine monochloride across the resulting alkene producedazide 1.7. Both the alcohol and amine were reprotected with benzoyl chloride, and the iodide was displaced with metachlorobenzoic acid in an oxidative nucleophilic substitution reaction to yield penultimate intermediate 1.9. All protecting
groups were then removed with ammonia in MeOH to yield
azvudine (1).
(6) Sun, L.; Peng, Y.; Yu, W.; Zhang, Y.; Liang, L.; Song, C.; Hou, J.;
Qiao, Y.; Wang, Q.; Chen, J.; et al. Mechanistic insight into
antiretroviral potency of 2’-deoxy-2’-beta-fluoro-4’-azidocytidine
(FNC) with a long-lasting effect on HIV-1 prevention. J. Med.
Chem. 2020, 63, 8554−8566.
(7) Chang, J. 4’-Modified nucleosides for antiviral drug discovery:
achievements and perspectives. Acc. Chem. Res. 2022, 55, 565−578.
(8) Zhang, J.-L.; Li, Y.-H.; Wang, L.-L.; Liu, H.-Q.; Lu, S.-Y.; Liu, Y.;
Li, K.; Liu, B.; Li, S.-Y.; Shao, F.-M.; Wang, K.; Sheng, N.; Li, R.; Cui,
J.-J.; Sun, P.-C.; Ma, C.-X.; Zhu, B.; Wang, Z.; Wan, Y.-H.; Yu, S.-S.;
Che, Y.; Wang, C.-Y.; Wang, C.; Zhang, Q.; Zhao, L.-M.; Peng, X.-Z.;
Cheng, Z.; Chang, J.-B.; Jiang, J.-D. Azvudine is a thymus-homing
anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal
Transduct. Target Ther. 2021, 6, 414.
(9) Chen, X.; Wang, Z.; Yu, H.; Liu, X. Preparation method of
azvudine and its intermediates. China Patent CN 115960147, 2023.
(10) Smith, D. B.; Kalayanov, G.; Sund, C.; Winqvist, A.; Maltseva,
T.; Leveque, V. J.-P.; Rajyaguru, S.; Pogam, S. L.; Najera, I.;
Benkestock, K.; Zhou, X.-X.; Kaiser, A. C.; Maag, H.; Cammack, N.;
Martin, J. A.; Swallow, S.; Johansson, N. G.; Klumpp, K.; Smith, M.
The design, synthesis, and antiviral activity of monofluoro and
difluoro analogues of 4’-azidocytidine against hepatitis C virus
replication: The discovery of 4’-azido-2’-deoxy-2’-fluorocytidine and4’-azido-2’-dideoxy-2’,2’-difluorocytidine. J. Med. Chem. 2009, 52,
2971−2978.
(11) Wang, Q.; Hu, W.; Wang, S.; Pan, Z.; Tao, L.; Guo, X.; Qian,
K.; Chen, C. H.; Lee, K. H.; Chang, J. Synthesis of new 2’-deoxy-2’-
fluoro-4’-azido nucleoside analogues as potent anti-HIV agents. Eur. J.
Med. Chem. 2011, 46, 4178−83.
(12) Deng, W.; Jiang, S.; Yu, T.; Zhai, D. Synthesis method of
azvudine. China Patent CN 111892636, 2020.
Medical uses
In July 2021, azvudine became conditionally approved in China for the following indication: “to treat high-viral-load cases of HIV-1, in combination with a nucleoside reverse-transcriptase inhibitor and a non-nucleoside reverse-transcriptase inhibitor”. The approval text describes it as a dual reverse transcriptase and Vif inhibitor.[10]
In July 2022, azvudine received emergency conditional approval for COVID-19 in adults.[11] It is believed to work by inhibiting the RNA-dependent RNA polymerase (RdRp) enzyme in the SARS-CoV-2 virus.[12][13]
Adverse effects
According to the manufacturer, phase II trials of azvudine in combination with doravirine and tenofovir disoproxil fumarate in HIV patients found an adverse effect profile similar to, but milder, than lamivudine combined with the two drugs. Very common (> 10%) side effects include dizziness, elevated liver enzymes, vomiting, and elevated alkaline phosphatase. Common (> 1%) side effects include nausea, elevated blood lipids, fever, insomnia, tiredness, and diarrhea. Detailed numbers are provided by Genuine in the slides and the medication package insert.[14][15] A boxed warning is present at the beginning of the Chinese package insert, describing a risk of “decrease in absolute neutrophil count, increase in total bilirubin, increase in glutathione aminotransferase, and increase in blood glucose”.[15]
The small (n=10) open-label pilot study for azvudine used alone in COVID reported no adverse events.[16]
Non-human models
Azvudine is found to be mutagenic in in vitro in the Ames test, CHL test, and in vitro in the mice micronucleus test.[17]
Azvudine is toxic to the reproductive system of rats and rabbit. The minimum reproductive NOAEL found for males is 5.0 mg/kg/d and for females 0.5 mg/kg/d. It is excreted in rat breast milk; the NOAEL for rat pups is 1.5 mg/kg/d.[17]
Azvudine is mainly toxic to the immune system, bone marrow, and digestive system of model animals. The chronic NOAELs are 0.5 mg/kg/d (rat, 3 months), 0.3 mg/kg/d (rat, 26 weeks), and 0.1 mg/kg/d (beagle dog, 1 month and 39 weeks).[17] For comparison, the chronic human dose for HIV treatment is 0.05 mg/kg/d, using the reference 3 mg dose and an average Chinese body mass of 59.5 kg (2014).
History
Azvudine was first found in literature in a patent filed by Chang Jun-biao of Zhengzhou University.[8] It received its current name in 2009, when researchers at Roche independently discovered it as a Hep C RNA polymerase inhibitor in vitro.[4] In the following years, Chinese scientists tested it in vitro for a number of targets, most importantly HBV (human and duck) and HTLV-1, two viruses with a reverse transcriptase.[18][19][20]
It was first proposed as an HIV treatment in 2011, when in vitro tests by the Chang group provided positive results.[21] In 2014, its oral pharmokinetics in rats was elucidated.[1] A phase II study (NCT04109183) was finished in March 2019 by Genuine Biotech. In August 2020, the Chang group found that the substance inhibits vif in vitro.[22] In the same month, China’s drug regulator (NMPA) decided to fast-track the approval process, labelling it a first-in-class medication.[14] In July 2021, NMPA granted conditional approval for HIV-1.[7] It was included in the 2021 HIV treatment recommendnation by the Chinese Medical Association and Chinese CDC, published October that year.[14] Curiously, no full results of the trial have been made available for this study in any journal detailing the experiment design as of December 2022.[23] Parts of the results are shown on the drug monograph as well as a 2022 slides deck produced by Genuine for the NHSA available on the latter’s website.[14]
Azvudine was found to inhibit some coronaviruses in vitro around 2020, leading to an interest in its use in COVID. An open-label pilot study on mild and moderate cases was performed in 2020, with mildly positive results.[16] A phase III trial was performed in 2022 in China. In July 2022, China’s drug regulator granted conditional approval for it to be used to treat COVID-19, following a local phase III trial.[6] Initially, no detailed description of the said trial was published in any journals, but state media quoted some numbers from the developer: “40% clinical improvement in 7 days by FNC group, compared to 11% in control”.[7] It is unclear how such “improvement” is defined.
Four phase III clinical trials investigated azvudine’s efficacy and safety in adults with mild-to-moderate COVID-19. The findings indicate that azvudine may reduce the time to eliminate detectable levels of virus (viral load) and improve symptoms faster than standard treatment. In trials, it was reported to be safe with few side effects. However, some studies produced inconsistent results in terms of symptom improvement and severe illness prevention. Additionally, the studies tended to use a smaller number of participants than other major COVID-19 drug trials.[12][13]
Society and culture
Genuine owns two different tradenames for this medication: 双新艾克 (literally “dual new AIDS inhibitor”) for HIV use[14] and 捷倍安 (literally “fast extra safe”) for COVID use.[7] No generics are available.
Geniune holds one patent related to the drug: the original 2007 patent on the entire class of 2′-fluorine-4′-substituted nucleotides, purchased from Zhengzhou University.[8] Two other Chinese patents on synthesizing the drug are found on Google Patents, but the owners do not appear to be connected to Geniune.[24] Roche held one 2002 patent, CNA028118480A (CN1516590A), over the broader class of 4′-substituted nucleotides. The patent was voided in 2019 after Riboscience, its new holder, stopped paying fees.[25]
References
- Peng Y, Cheng T, Dong L, Zhang Y, Chen X, Jiang J, et al. (September 2014). “Quantification of 2′-deoxy-2′-β-fluoro-4′-azidocytidine in rat and dog plasma using liquid chromatography-quadrupole time-of-flight and liquid chromatography-triple quadrupole mass spectrometry: Application to bioavailability and pharmacokinetic studies”. Journal of Pharmaceutical and Biomedical Analysis. 98: 379–386. doi:10.1016/j.jpba.2014.06.019. PMID 24999865.
- Liu Y, Liu B, Zhang Y, Peng Y, Huang C, Wang N, et al. (July 2017). “Intestinal absorption mechanisms of 2′-deoxy-2′-β-fluoro-4′-azidocytidine, a cytidine analog for AIDS treatment, and its interaction with P-glycoprotein, multidrug resistance-associated protein 2 and breast cancer resistance protein”. European Journal of Pharmaceutical Sciences. 105: 150–158. doi:10.1016/j.ejps.2017.05.009. PMID 28487144. S2CID 4252337.
- Wang RR, Yang QH, Luo RH, Peng YM, Dai SX, Zhang XJ, et al. (2014). “Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro”. PLOS ONE. 9 (8): e105617. Bibcode:2014PLoSO…9j5617W. doi:10.1371/journal.pone.0105617. PMC 4140803. PMID 25144636.
- Smith DB, Kalayanov G, Sund C, Winqvist A, Maltseva T, Leveque VJ, et al. (May 2009). “The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4′-azidocytidine against hepatitis C virus replication: the discovery of 4′-azido-2′-deoxy-2′-fluorocytidine and 4′-azido-2′-dideoxy-2′,2′-difluorocytidine”. Journal of Medicinal Chemistry. 52 (9): 2971–2978. doi:10.1021/jm801595c. PMID 19341305.
- Harrison C (April 2020). “Coronavirus puts drug repurposing on the fast track”. Nature Biotechnology. 38 (4): 379–381. doi:10.1038/d41587-020-00003-1. PMID 32205870.
- Ye Y (July 2022). “China approves first homegrown COVID antiviral”. Nature. doi:10.1038/d41586-022-02050-x. PMID 35883009. S2CID 251104078.
- “首个国产抗新冠口服药附条件获批上市” [First domestic oral anti-Covid drug conditionally approved]. 新华网. 证券日报. 2022-07-26. Archived from the original on 2022-08-09. Retrieved 2022-07-26.
- Chang J, Bao X, Wang Q, Guo X, Wang W, Qi X. Preparation of 2′-fluoro-4′-substituted nucleoside analogs as antiviral agents. 20070807. Chinese Patent Application No: CN 2007-10137548. Chinese Patent No: CN 101177442A, 20080514.
- “新冠口服药阿兹夫定片线上开售, 每瓶售价350元” [Oral COVID drug Azvudine tablet available online at 350 yuan/bottle]. Xinmin Evening News. 19 November 2022. Retrieved 28 December 2022 – via Beijing Daily (repost).
- “国家药监局附条件批准阿兹夫定片上市” [NMPA conditionally approvals azvudine tablets]. www.nmpa.gov.cn (in Chinese). 2021-07-21.
- “国家药监局应急附条件批准河南真实生物科技有限公司阿兹夫定片增加新冠肺炎治疗适应症注册申请” [NMPA grants emergency conditional approval for additional indication registration of azvudine tablets (Hebei Genuine Biotech Co., Ltd.)]. www.nmpa.gov.cn. 2022-07-25.
- Zhu KW (2023). “Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials”. Frontiers in Pharmacology. 14: 1228548. doi:10.3389/fphar.2023.1228548. PMC 10484631. PMID 37693894.
- Wang Y, Xie H, Wang L, Fan J, Zhang Y, Pan S, Zhou W, Chen Q, Liu X, Wu A, Zhang H, Wang J, Tian X (February 2024). “Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis”. Virology Journal. 21 (1): 46. doi:10.1186/s12985-024-02316-y. PMC 10893615. PMID 38395970.
- Genuine Biotech (July 11, 2022). “阿兹夫定片(双新艾克)” [Azvudine Tablets (Shuāngxīnàikè)]. NHSA.gov.cn. Archived from the original on 2022-09-06.
- “阿兹夫定片说明书” [Azvudine Tablets, Monograph] (PDF). WUXU DATA. Retrieved 2023-01-03.
- Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, et al. (October 2020). “A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study”. Advanced Science. 7 (19): e2001435. doi:10.1002/advs.202001435. PMC 7404576. PMID 35403380.
- Drug Review Center (China) (June 30, 2022). “阿兹夫定片 (CXHS2000016-17) 申请上市技术审评报告” [Azvudine tabs (CHXS2000016-17) request for marketing technical review report] (PDF). WUXU DATA. Retrieved 2023-01-03.
- Wang Q, Liu X, Wang Q, Zhang Y, Jiang J, Guo X, et al. (April 2011). “FNC, a novel nucleoside analogue inhibits cell proliferation and tumor growth in a variety of human cancer cells”. Biochemical Pharmacology. 81 (7): 848–855. doi:10.1016/j.bcp.2011.01.001. PMID 21219886.
- Zheng L, Wang Q, Yang X, Guo X, Chen L, Tao L, et al. (2012). “Antiviral activity of FNC, 2′-deoxy-2′-β-fluoro-4′-azidocytidine, against human and duck HBV replication”. Antiviral Therapy. 17 (4): 679–687. doi:10.3851/IMP2094. PMID 22452880. S2CID 25576607.
- Zhou Y, Zhang Y, Yang X, Zhao J, Zheng L, Sun C, et al. (2012). “Novel nucleoside analogue FNC is effective against both wild-type and lamivudine-resistant HBV clinical isolates”. Antiviral Therapy. 17 (8): 1593–1599. doi:10.3851/IMP2292. PMID 22910281. S2CID 29382902.
- Wang Q, Hu W, Wang S, Pan Z, Tao L, Guo X, et al. (September 2011). “Synthesis of new 2′-deoxy-2′-fluoro-4′-azido nucleoside analogues as potent anti-HIV agents”. European Journal of Medicinal Chemistry. 46 (9): 4178–4183. doi:10.1016/j.ejmech.2011.06.020. PMC 3164908. PMID 21745701.
- Sun L, Peng Y, Yu W, Zhang Y, Liang L, Song C, et al. (August 2020). “Mechanistic Insight into Antiretroviral Potency of 2′-Deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a Long-Lasting Effect on HIV-1 Prevention”. Journal of Medicinal Chemistry. 63 (15): 8554–8566. doi:10.1021/acs.jmedchem.0c00940. PMID 32678592. S2CID 220631451.
- Li G, Wang Y, De Clercq E (April 2022). “Approved HIV reverse transcriptase inhibitors in the past decade”. Acta Pharmaceutica Sinica. B. 12 (4): 1567–1590. doi:10.1016/j.apsb.2021.11.009. PMC 9279714. PMID 35847492.
- Google Patents Search, “阿兹夫定” (with quotes), CN114149475A, CN111892636A.
- Guokr.com (10 August 2022). “真实生物的真实面目”. Huxiu.com. Retrieved 30 December 2022.
Further reading
- Zhu KW (2023). “Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials”. Frontiers in Pharmacology. 14: 1228548. doi:10.3389/fphar.2023.1228548. PMC 10484631. PMID 37693894.
| Clinical data | |
|---|---|
| Trade names | 捷倍安, 双新艾克 |
| Other names | 2′-Deoxy-2′-β-fluoro-4′-azidocytidine (FNC), RO-0622 |
| Legal status | |
| Legal status | US: Investigational drugCN: Conditional use Rx |
| Pharmacokinetic data | |
| Bioavailability | 83% (rat, dog)[1] |
| Metabolism | liver (CYP3A)[2] |
| Elimination half-life | 4 hours (dog)[1] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1011529-10-4 |
| PubChem CID | 24769759 |
| DrugBank | DB16407 |
| ChemSpider | 24717759 |
| UNII | IJ2XP0ID0K |
| ChEMBL | ChEMBL519846 |
| Chemical and physical data | |
| Formula | C9H11FN6O4 |
| Molar mass | 286.223 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
///////AZVUDINE, Genuine Biotech, APPROVALS 2022, CHINA 2022, FNC, HY 19314, RO 0622, RO-0622, SB17040, IJ2XP0ID0K, DTXSID901027757



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……














