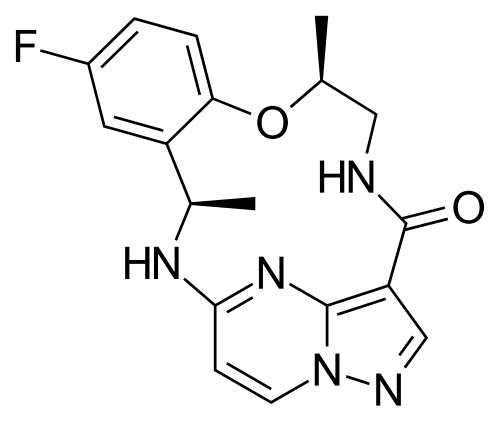
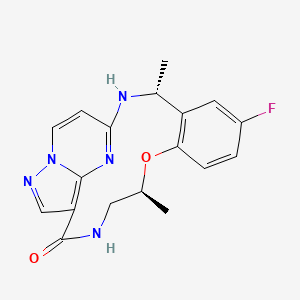
Ropotrectinib
- CAS 1802220-02-5
- TPX-0005
- Augtyro
- 08O3FQ4UNP
WeightAverage: 355.373
Monoisotopic: 355.144453003
Chemical FormulaC18H18FN5O2
- repotrectinibum
- (3R,11S)-6-fluoro-3,11-dimethyl-10-oxa-2,13,17,18,21-pentazatetracyclo[13.5.2.04,9.018,22]docosa-1(21),4(9),5,7,15(22),16,19-heptaen-14-one
- 1,15-Etheno-1H-pyrazolo(4,3-F)(1,4,8,10)benzoxatriazacyclotridecin-4(5H)-one, 11-fluoro-6,7,13,14-tetrahydro-7,13-dimethyl-, (7S,13R)-
- 1,15-Etheno-1H-pyrazolo(4,3-f)(1,4,8,10)benzoxatriazacyclotridecin-4(5H)-one, 11-fluoro-2,6,7,13-tetrahydro-7,13-dimethyl-, (14Z)-
- (1Z)-6-Fluoro-3,11-dimethyl-10-oxa-2,13,17,18,21-pentaazatetracyclo(13.5.2.04,9.018,22)docosa-1,4,6,8,15,19,21-heptaen-14-one
- (3R,11S)-6-fluoro-3,11-dimethyl-10-oxa-2,13,17,18,21-pentaazatetracyclo[13.5.2.0,.0,]docosa-1(21),4(9),5,7,15(22),16,19-heptaen-14-one
- (7S,13R)-11-fluoro-7,13-dimethyl-6,7,13,14- tetrahydro-1,15-ethenopyrazolo[4,3- f][1,4,8,10]benzoxatriazacyclotridecin-4(5H)- one
- (3R,6S,)-45-FLUORO-3,6-DIMETHYL-5-OXA-2,8-DIAZA-1(5,3)-PYRAZOLO(1,5-A)PYRIMIDINA-4(1,2)-BENZENANONAPHAN-9-ONE
- (7S,13R)-11-Fluoro-7,13-Dimethyl-6,7,13,14-Tetrahydro-1,15-Ethenopyrazolo[4,3-F][1,4,8,10]Benzoxatriazacyclotridecin-4(5H)-One
- 1,15-ETHENO-1H-PYRAZOLO(4,3-F)(1,4,8,10)BENZOXATRIAZACYCLOTRIDECIN-4(5H)-ONE, 11-FLUORO-6,7,13,14-TETRAHYDRO-7,13-DIMETHYL-, (7S,13R)-
Repotrectinib, sold under the brand name Augtyro, is an anti-cancer medication used for the treatment of non-small cell lung cancer.[2][5] It is taken by mouth.[2] Repotrectinib is an inhibitor of proto-oncogene tyrosine-protein kinase ROS1 (ROS1) and of the tropomyosin receptor tyrosine kinases (TRKs) TRKA, TRKB, and TRKC.[2]
The most common adverse reactions include dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, ataxia, fatigue, cognitive disorders, and muscular weakness.[5]
Repotrectinib was approved for medical use in the United States in November 2023,[5][6] and in the European Union in January 2025.[3][4] CHINA 2024
SYN
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.202405153
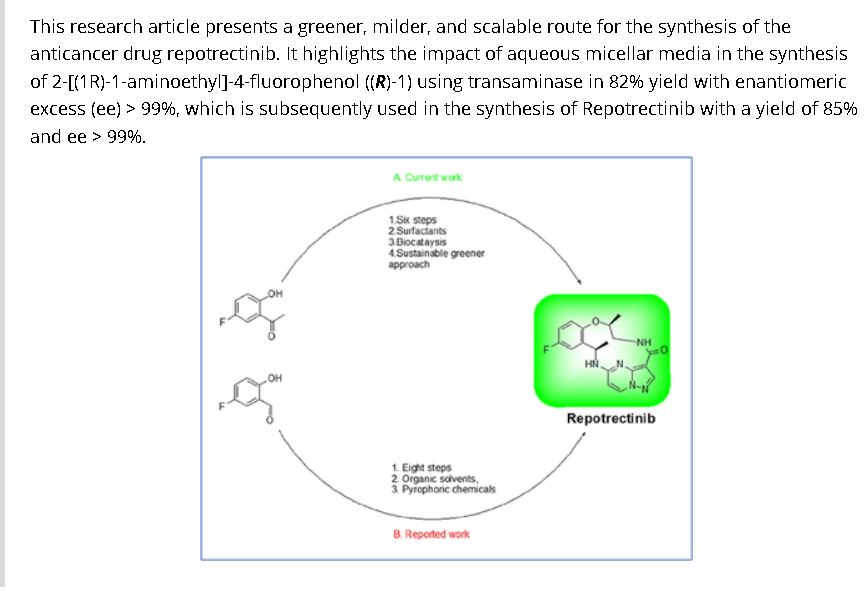
Synthesis of Repotrectinib
To a stirred solution of 5-{[(1R)-1-(2-{[(2S)-1-aminopropan-2-yl]oxy}-5-fluorophenyl)ethyl]amino}pyrazolo[1,5-a]pyrimidine-3-carboxylic acid 15 (0.25 g, 0.000611 mol, 1.0 eq.) in DMF (4.0 mL, 16V) was slowly added to solution of DIPEA (0.6 mL, 0.00488 mol, 8.0 eq.) in DCM (1.8 mL, 7V) at 0-5 °C. Then FDPP (0.25 g, 0.000672 mol, 1.1 eq.) was added at 0-5 °C. The reaction mixture was allowed to stirr for 1-2h at 25-30 °C. The reaction was monitored by TLC for disappearance of starting material. Then the resulting reaction mixture was diluted with ethyl acetate (50 mL), washed with water (20 mL) and brine solution (20 mL). The separated organic layer was dried over sodium sulphate and concentrated under reduced pressure at 45 °C. The obtained crude product was purified by silica gel (60-120 mesh) column chromatography to get repotrectinib asawhite solid (0.18 g, 85%).
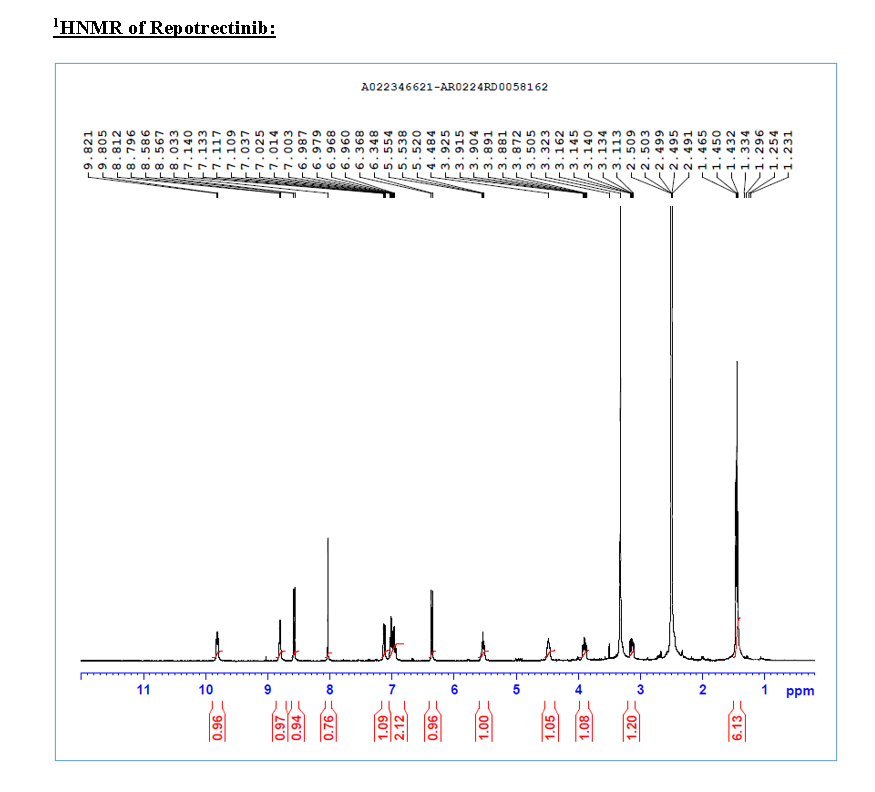
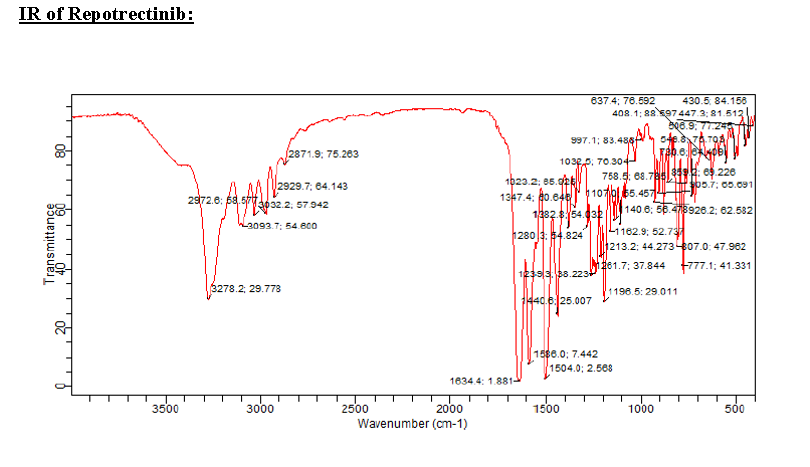
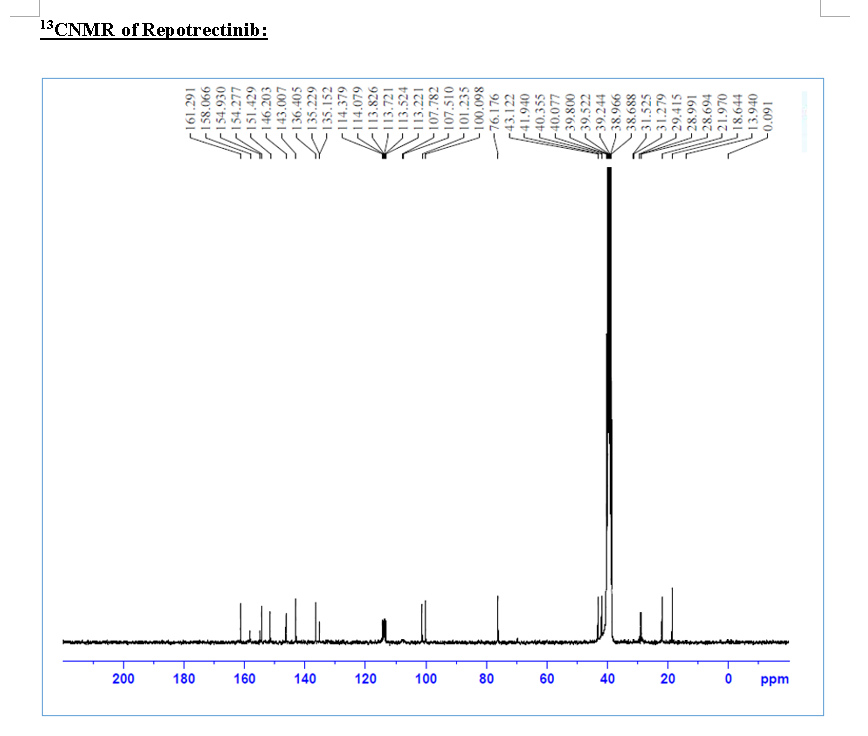
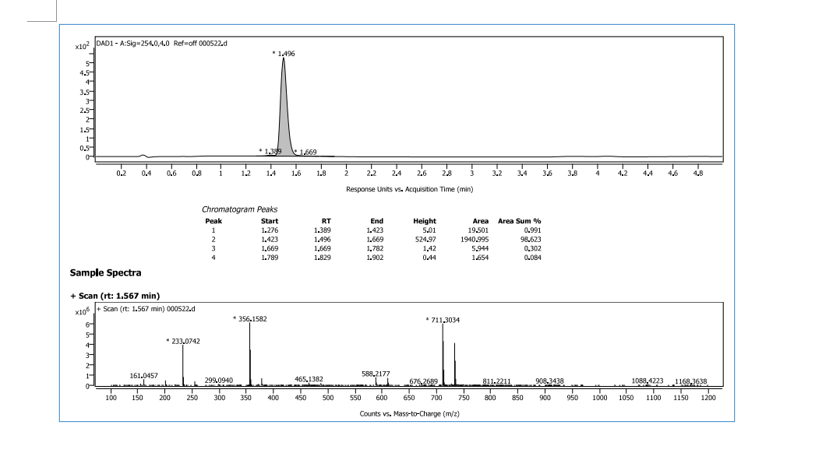
HRMS
SYN
https://pubs.acs.org/doi/10.1021/acs.oprd.3c00152
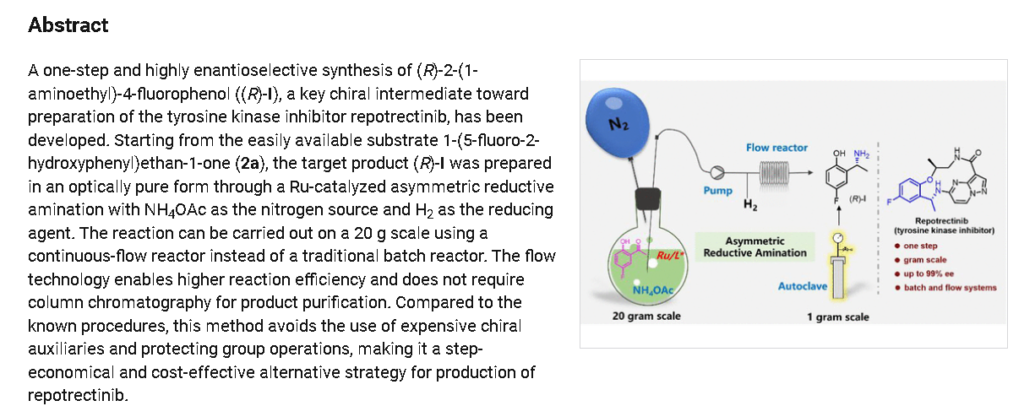
REF
https://pubs.acs.org/doi/10.1021/acs.oprd.4c00061
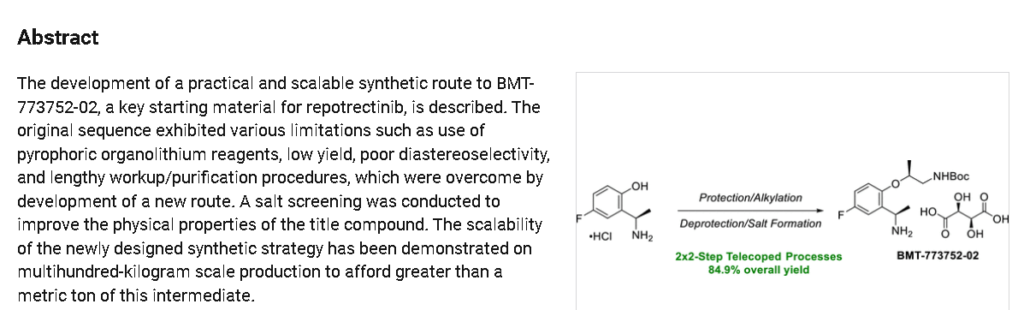
REF
US20180194777
https://patentscope.wipo.int/search/en/detail.jsf?docId=US222923082&_cid=P11-ME283N-03701-1
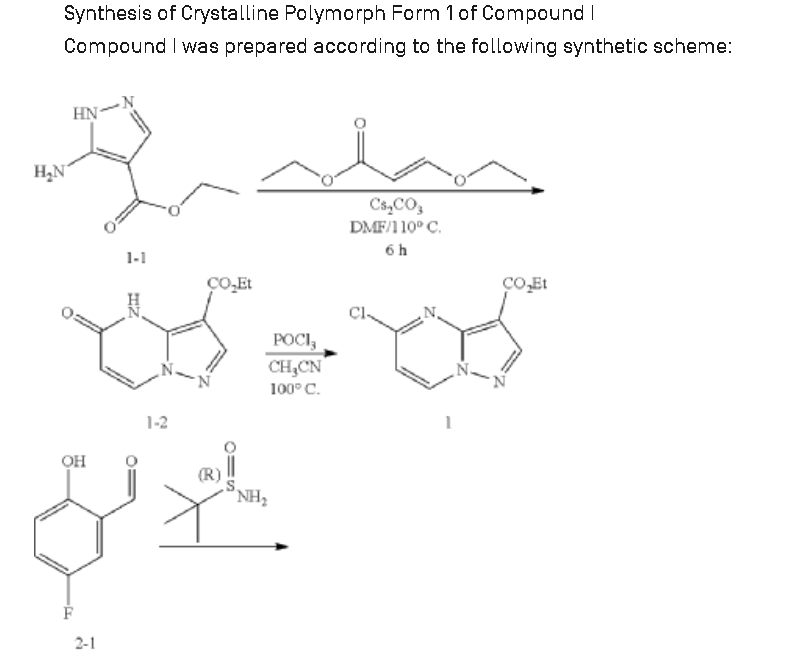
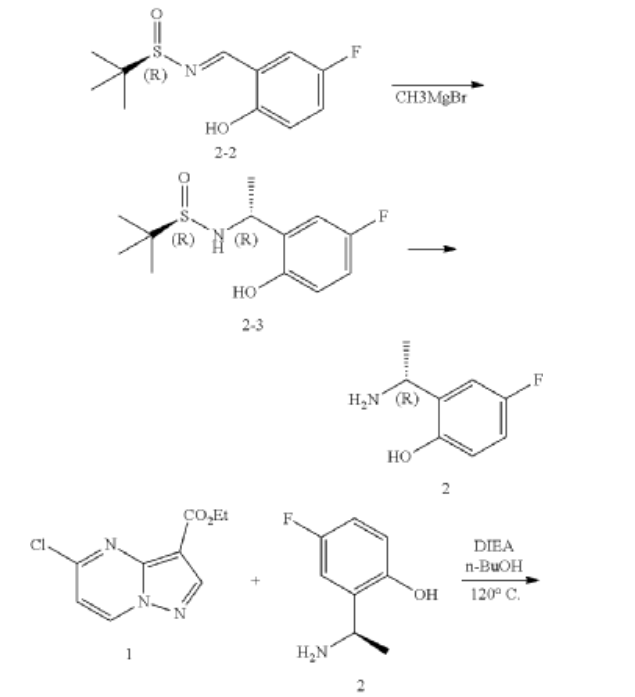
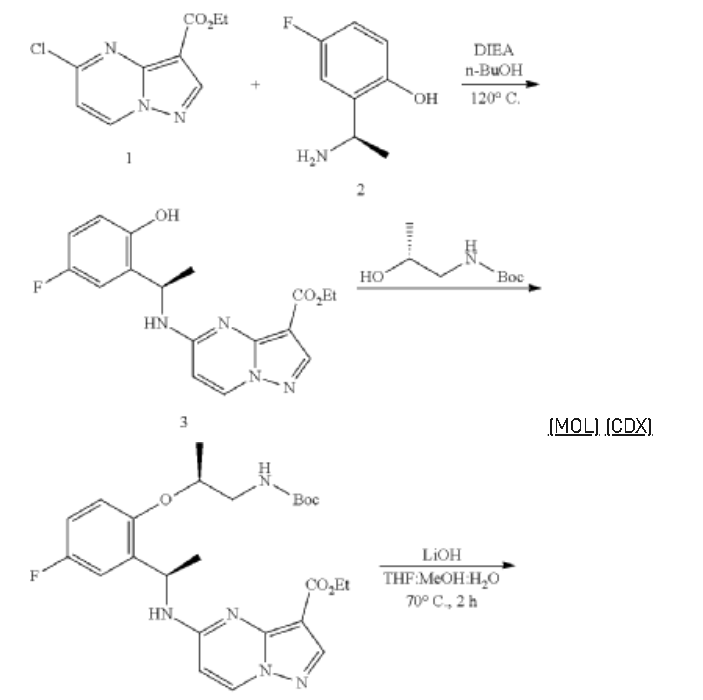
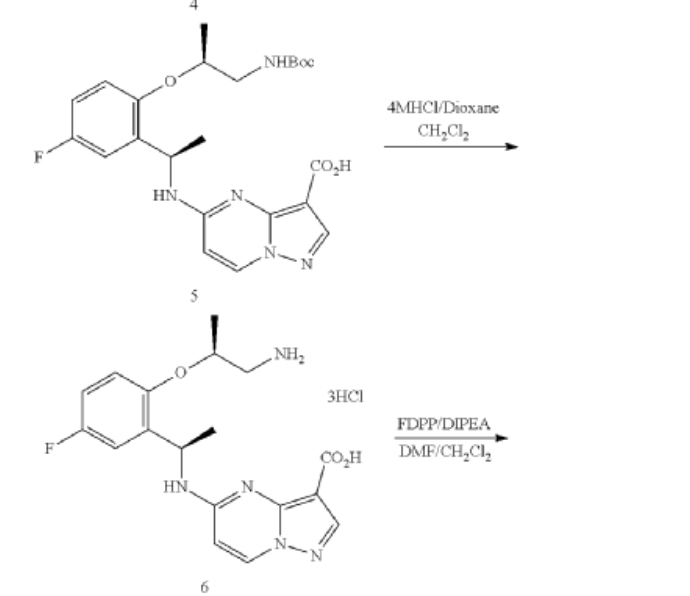
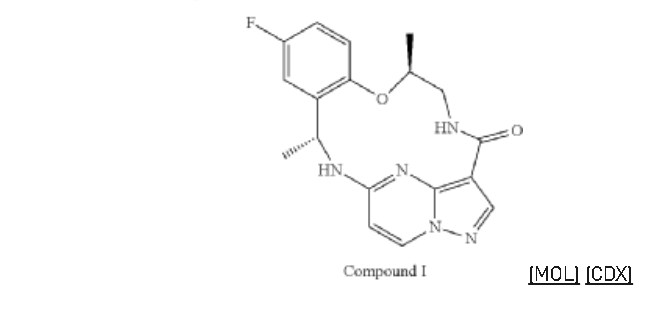
Example 1: Preparation of 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (1)
Step 1: Preparation of ethyl 5-oxo-4H-pyrazolo[1,5-a]pyrimidine-3-carboxylate (1-2)
Step 2: Preparation of 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (1)
PATENT
https://patents.google.com/patent/US10246466B2/en

Step 1. To a solution of tert-butyl (R)-(2-hydroxypropyl)carbamate (1.00 g, 5.71 mmol) and tosyl chloride (1.14 g, 6.00 mmol) in DCM (29 mL) was added triethylamine (1.44 g, 14.28 mmol and the mixture was stirred at room temp for 48 hour. The reaction solution was concentrated under reduced pressure and the residue was purified with flash chromatography (ISCO system, silica (40 g), 0-20% ethyl acetate in hexane) to provide (R)-1-((tert-butoxycarbonyl)amino)propan-2-yl 4-methylbenzenesulfonate (1.12 g, 3.40 mmol, 59.54% yield).
Step 2. To a solution of A8 (100.00 mg, 0.290 mmol) and (R)-1-((tert-butoxycarbonyl)amino)propan-2-yl 4-methylbenzenesulfonate (143.50 mg, 0.436 mmol) in DMF (1.45 mL) was added K2CO3 (200.7 mg, 1.45 mmol) and heated at 80° C. with stirring for 16 hour. The reaction was cooled to ambient temperature and diluted with DCM (3 mL), filtered through a syringe filter, and concentrated under reduced pressure. Flash chromatography (ISCO system, silica (12 g), 0-60% ethyl acetate in hexane) provided 93A (32.90 mg, 0.0656 mmol, 22.59% yield).
Step 3. To a solution of 93A (32.90 mg, 0.0656 mmol) in MeOH (3 mL) and THF (2 mL) was added LiOH aqueous solution (2M, 2 mL) at ambient temperature. The reaction solution was heated at 70° C. for 2 hours The reaction flask was cooled to ambient temperature, diluted with water and methanol, and then quenched with HCl aqueous solution (2 M, 2 mL) to pH<5. The mixture was extracted with DCM (3×5 mL), dried with Na2SO4, concentrated under reduced and dried on high vacuum overnight. To a solution of the acid product in DCM (4 mL) was added 4 M HCl in 1,4-dioxane (2.0 mL). The mixture was stirred at room temperature for 3 hours, and then concentrated under reduced pressure and dried on high vacuum. To a solution of the de-Boc product and FDPP (27.62 mg, 0.0719 mmol) in DMF (1.6 mL) was added Hunig’s base (42.23 mg, 0.327 mmol) at room temperature. The mixture was stirred for 2.5 hours, and then quenched the reaction with 2 M Na2CO3 solution (2 mL). The mixture was stirred for 15 min then extracted with DCM (4×10 mL). The combined extracts were dried with Na2SO4 and concentrated under reduced pressure. The residue was purified with flash chromatography (ISCO system, silica (12 g), 0-10% methanol in dichloromethane) to provide 93 (10.1 mg, 0.0284 mmol, 43.49% yield for three steps).
PATENT
SYN
European Journal of Medicinal Chemistry 265 (2024) 116124
Repotrectinib (Augtyro) Repotrectinib, developed by Turning Point Therapeutics, Inc., was granted FDA approval on November 15, 2023. It is indicated to treat locally advanced or metastatic ROS proto-oncogene 1, receptor tyrosine kinase (ROS1)-positive non-small cell lung cancer (NSCLC). Repotrectinib is a highly effective inhibitor of ROS1 (ICtyrosine receptor kinase (TRK) (IC5050= 0.07 nM) and
=0.83/0.05/0.1 nM for TRKA/B/C) [87]. After undergoing currently approved targeted therapies, patients with tumors containing ROS1 and neurotrophic tyrosine kinase receptor (NTRK) gene fusions frequently acquire resistance mutations [88,89]. These mutations restrict the ability of drugs to bind to their
targets, ultimately resulting in the advancement of tumors. Repotrectinib, a novel tyrosine kinase inhibitor (TKI), is the pioneering drug developed to specifically target ROS1 or NTRK-positive metastatic
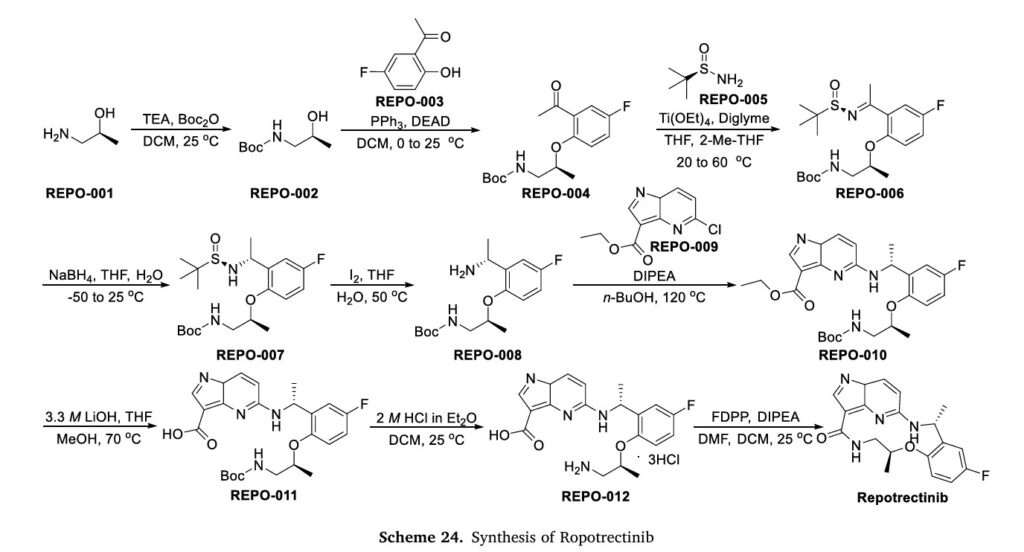
NSCLC and effectively combat the primary factors contributing to disease advancement [90].Preparation of Repotrectinib is described as Scheme 24 [91].Protecting the amino group of REPO-001 with Boc group in the presence of Kgave REPO-002, followed by intermolecular dehydration with
1-(5-fluoro-2-hydroxyphenyl)ethan-1-one (REPO-003) to give the ester REPO-004. REPO-004 was reacted with chiral auxiliary REPO-005 to give REPO-006, which was reduced by NaBH4
to obtain REPO-007. Then REPO-008 was obtained by removing the chiral auxiliary under iodine conditions. Substitution of REPO-008 with REPO-009 gave REPO-010, which was further hydrolyzed under alkaline conditions to obtain REPO-011. Salt formation of REPO-011 with hydrochloric acid
yielded REPO-012, which underwent intramolecular condensation to obtain the product Repotrectinib.
[87] D. Zhai, W. Deng, Z. Huang, E. Rogers, J.J. Cui, The novel, rationally-designed,
ALK/SRC inhibitor TPX-0005 overcomes multiple acquired resistance
mechanisms to current ALK inhibitors, Cancer Res. 76 (2016) 2132.
[88] C. Keddy, P. Shinde, K. Jones, S. Kaech, R. Somwar, U. Shinde, M.A. Davare,
Resistance profile and structural modeling of next-generation ROS1 tyrosine
kinase inhibitors, Mol. Cancer Therapeut. 21 (2022) 336–346.
[89] E. Cocco, M. Scaltriti, A. Drilon, NTRK fusion-positive cancers and TRK inhibitor
therapy, Nat. Rev. Clin. Oncol. 15 (2018) 731–747.
[90] A. Drilon, S.I. Ou, B.C. Cho, D.W. Kim, J. Lee, J.J. Lin, V.W. Zhu, M.J. Ahn, D.
R. Camidge, J. Nguyen, D. Zhai, W. Deng, Z. Huang, E. Rogers, J. Liu, J. Whitten,
J.K. Lim, S. Stopatschinskaja, D.M. Hyman, R.C. Doebele, J.J. Cui, A.T. Shaw,
Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that
potently inhibits ROS1/TRK/ALK solvent-front mutations, Cancer Discov. 8
(2018) 1227–1236.
[91] J.J. Cui, E.W. Rogers, Gialir Macrocyclic Polymorph, 2018. US20180194777A1.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
Syn
European Journal of Medicinal Chemistry 291 (2025) 117643
Repotrectinib, developed by Bristol-Myers Squibb and marketed under the brand name Augtyro, is an oral tyrosine kinase inhibitor (TKI) targeting ROS1 and TRK oncogenic drivers. In 2024, NMPA condition
ally approved Repotrectinib for adult patients with ROS1-positive locally advanced or metastatic NSCLC [15]. Repotrectinib exerts its antitumor activity by inhibiting ROS1 and TRK kinases, thereby disrupting the downstream signaling pathways that facilitate tumor cell proliferation and survival [16]. This argeted mechanism is particularly effective against tumors that harbor ROS1 or NTRK gene fusions. The clinical efficacy of Repotrectinib has been through validated the Phase 1/2 TRIDENT-1 trial (NCT03093116) [17]. In the study cohort, treat ment-naïve patients harboring ROS1-positive NSCLC exhibited an overall response rate (ORR) of 79 %, characterized by a median duration of response (DOR) reaching 34.1 months. Conversely, among those who had previously received ROS1 TKI therapy, the ORR was documented at 38 %, accompanied by a median DOR of 14.8 months. With respect to safety profiles, the adverse event spectrum commonly encompassed dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, fatigue, ataxia, cognitive impairment, muscular weakness, and nausea
[18,19]. These side effects are generally manageable, but patients should be monitored for potential severe adverse events.
The synthetic route of Repotrectinib, shown in Scheme 4, begins with condensation reaction between Repo-001 and Repo-002 to afford Repo-003, which is chlorinated to yield Repo-004 [20]. This intermediate undergoes nucleophilic substitution with Repo-005 to form Repo-006,
followed by second nucleophilic substitution with Repo-007 to produce Repo-008. Ester hydrolysis of Repo-008 affords Repo-009, which undergoes acid-mediated deprotection to generate Repo-010. Final
intramolecular amidation of Repo-010 delivers Repotrectinib. In parallel, Repo-011 and Repo-012 undergo condensation to form imine Repo-013, which undergoes Grignard addition to afford Repo-014.
Acidification of Repo-014 then yields Repo-005. Concurrently, Repo-015 undergoes nucleophilic substitution to generate Repo-007.
[15] S. Dhillon, Repotrectinib: first approval, Drugs 84 (2024) 239–246.
[16] T. Rais, A. Shakeel, L. Naseem, N. Nasser, M. Aamir, Repotrectinib: a promising
new therapy for advanced nonsmall cell lung cancer, Ann Med Surg (Lond) 86
(2024) 7265–7269.
[17] A. Drilon, S.I. Ou, B.C. Cho, D.W. Kim, J. Lee, J.J. Lin, V.W. Zhu, M.J. Ahn, D.
R. Camidge, J. Nguyen, D. Zhai, W. Deng, Z. Huang, E. Rogers, J. Liu, J. Whitten, J.
K. Lim, S. Stopatschinskaja, D.M. Hyman, R.C. Doebele, J.J. Cui, A.T. Shaw,
Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that
potently inhibits ROS1/TRK/ALK solvent-front mutations, Cancer Discov. 8 (2018)
1227–1236.
[18] Repotrectinib, Drugs and Lactation Database (Lactmed®), National Institute of
Child Health and Human Development, Bethesda (MD), 2006.
[19] H. Zhong, J. Lu, M. Wang, B. Han, Real-world studies of crizotinib in patients with
ROS1-positive non-small-cell lung cancer: experience from China, J Comp Eff Res
14 (2024) e240043.
[20] J.J. Cui, E.W. Rogers, Preparation of
Fluorodimethyltetrahydroethenopyrazolobenzoxatriazacyclotridecinone
Derivatives for Use as Antitumor Agents, 2017. US20180194777A1.

Repotrectinib is indicated for the treatment of adults with locally advanced or metastatic ROS1-positive non-small cell lung cancer.[2][5]
In June 2024, the US Food and Drug Administration (FDA) expanded the indication to include the treatment of people twelve years of age and older with solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, are locally advanced or metastatic or where surgical resection is likely to result in severe morbidity, and that have progressed following treatment or have no satisfactory alternative therapy.[7][8]
References
- “Register of Innovative Drugs”. Health Canada. 3 November 2006. Retrieved 23 May 2025.
- “Augtyro- repotrectinib capsule”. DailyMed. 15 November 2023. Archived from the original on 12 December 2023. Retrieved 12 December 2023.
- “Augtyro EPAR”. European Medicines Agency (EMA). 14 November 2024. Retrieved 16 November 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- “Augtyro PI”. Union Register of medicinal products. 14 January 2025. Retrieved 16 January 2025.
- “FDA approves repotrectinib for ROS1-positive non-small cell lung cancer”. U.S. Food and Drug Administration (FDA). 15 November 2023. Archived from the original on 16 November 2023. Retrieved 17 November 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - “U.S. Food and Drug Administration Approves Augtyro (repotrectinib), a Next-Generation Tyrosine Kinase Inhibitor (TKI), for the Treatment of Locally Advanced or Metastatic ROS1-Positive Non-Small Cell Lung Cancer (NSCLC)” (Press release). Bristol Myers Squibb. 16 November 2023. Archived from the original on 16 November 2023. Retrieved 17 November 2023 – via Business Wire.
- “FDA grants accelerated approval to repotrectinib for adult and pediatric participants with neurotrophic tyrosine receptor kinase gene fusion-positive solid tumors”. U.S. Food and Drug Administration. 13 June 2024. Archived from the original on 13 June 2024. Retrieved 13 June 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - “Cancer Accelerated Approvals”. U.S. Food and Drug Administration (FDA). 1 October 2024. Retrieved 6 December 2024.
- Turning Point Therapeutics, Inc. (5 February 2024). A Phase 1/2, Open-Label, Multi-Center, First-in-Human Study of the Safety, Tolerability, Pharmacokinetics, and Anti-Tumor Activity of TPX-0005 in Patients With Advanced Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements (TRIDENT-1) (Report). clinicaltrials.gov. Archived from the original on 18 June 2024. Retrieved 18 June 2024.
- “Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 11-14 November 2024”. European Medicines Agency (EMA). 15 November 2024. Retrieved 16 November 2024.
Further reading
- Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, et al. (October 2018). “Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations”. Cancer Discovery. 8 (10): 1227–1236. doi:10.1158/2159-8290.CD-18-0484. PMID 30093503.
External links
- “Repotrectinib (Code C133821)”. NCI Thesaurus. 25 September 2023. Retrieved 17 November 2023.
| Clinical data | |
|---|---|
| Trade names | Augtyro |
| Other names | TPX-0005 |
| AHFS/Drugs.com | Augtyro |
| License data | US DailyMed: Repotrectinib |
| Routes of administration | By mouth |
| Drug class | Tyrosine kinase inhibitor |
| ATC code | L01EX28 (WHO) |
| Legal status | |
| Legal status | CA: ℞-only[1]US: ℞-only[2]EU: Rx-only[3][4] |
| Identifiers | |
| CAS Number | 1802220-02-5 |
| PubChem CID | 135565923 |
| DrugBank | DB16826 |
| ChemSpider | 64853849 |
| UNII | 08O3FQ4UNP |
| KEGG | D11454 |
| ChEBI | CHEBI:229220 |
| ChEMBL | ChEMBL4298138 |
| PDB ligand | 7GI (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C18H18FN5O2 |
| Molar mass | 355.373 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
- (3R,6S,)-45-FLUORO-3,6-DIMETHYL-5-OXA-2,8-DIAZA-1(5,3)-PYRAZOLO(1,5-A)PYRIMIDINA-4(1,2)-BENZENANONAPHAN-9-ONE
- (7S,13R)-11-Fluoro-7,13-Dimethyl-6,7,13,14-Tetrahydro-1,15-Ethenopyrazolo[4,3-F][1,4,8,10]Benzoxatriazacyclotridecin-4(5H)-One
- 1,15-ETHENO-1H-PYRAZOLO(4,3-F)(1,4,8,10)BENZOXATRIAZACYCLOTRIDECIN-4(5H)-ONE, 11-FLUORO-6,7,13,14-TETRAHYDRO-7,13-DIMETHYL-, (7S,13R)-
////////Ropotrectinib, FDA 2023, APPROVALS 2023, Turning Point , EU 2025, APPROVALS 2025, EMA 2025, Augtyro, TPX 0005, CHINA 2024, APPROVALS 2024














