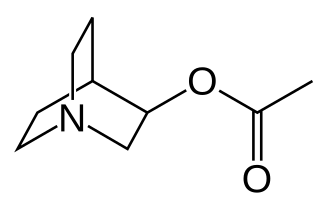
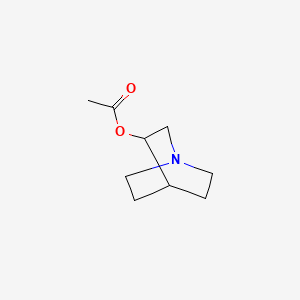
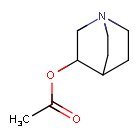
Aceclidine
WeightAverage: 169.224
Monoisotopic: 169.110278727
Chemical FormulaC9H15NO2
CAS 827-61-2, 3-Acetoxyquinuclidine, 3-Quinuclidinol acetate (ester), Aceclidina, 0578K3ELIO
APROVAL 7/31/2025, Vizz. To treat presbyopia
1-azabicyclo[2.2.2]octan-3-yl acetate
Acetic acid 1-aza-bicyclo[2.2.2]oct-3-yl ester(aceclidine)
MW: 169.22 MF: C9H15NO2
LD50: 78 mg/kg (M, i.p.); 36 mg/kg (M, i.v.); 165 mg/kg (M, p.o.); 102 mg/kg (M, s.c.);
45 mg/kg (R, i.v.); 225 mg/kg (R, s.c.)
CN: 1-azabicyclo[2.2.2]octan-3-ol acetate (ester)
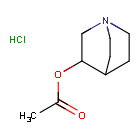
WeightAverage: 205.68
Monoisotopic: 205.0869565
Chemical FormulaC9H16ClNO2
LD50: 27 mg/kg (M, i.v.); 165 mg/kg (M, p.o.);
45 mg/kg (R, i.v.)
Aceclidine (Glaucostat, Glaunorm, Glaudin, Vizz) is a parasympathomimetic miotic agent used in the treatment of narrow angle glaucoma.
Aceclidine was approved for medical use in the United States in July 2025.[2]
Medicinal properties
Aceclidine decreases intraocular pressure. It acts as a muscarinic acetylcholine receptor agonist.[3]
Chemistry
Aceclidine is an organic compound that is structurally related to quinuclidine. As such its alternative name is 3-acetoxyquinuclidine. Its protonated derivative has a pKa of 9.3.[4]
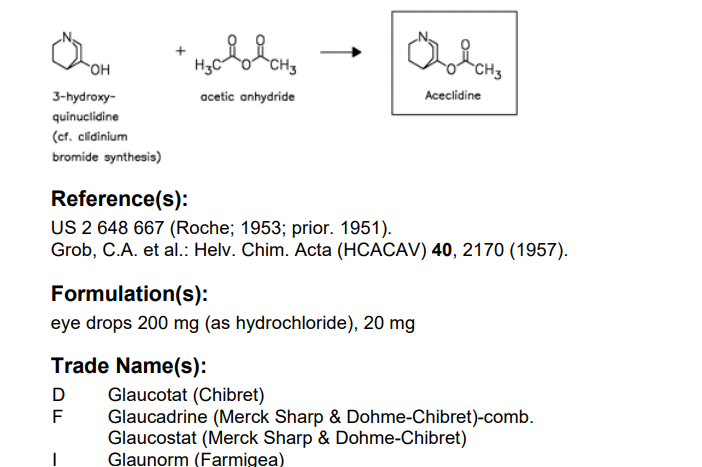
SYN
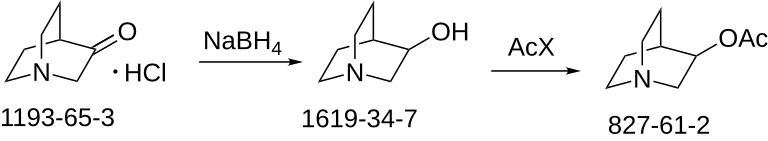
E. E. Mikhlina and M. V. Rubtsov, Zhur. Obschei
Khim, 30, 163 (1960). L. H. Sternbach and S. Kaiser, J. Am. Chem. Soc., 74, 2215 (1952). C. A. Grob, A. Kaiser and E. Renk, Helv. Chim.Acta, 40, 2170 (1957).



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/218585s000lbl.pdf
- “Novel Drug Approvals for 2025”. U.S. Food and Drug Administration (FDA). 4 August 2025. Retrieved 5 August 2025.
- Shannon HE, Hart JC, Bymaster FP, Calligaro DO, DeLapp NW, Mitch CH, et al. (August 1999). “Muscarinic receptor agonists, like dopamine receptor antagonist antipsychotics, inhibit conditioned avoidance response in rats”. The Journal of Pharmacology and Experimental Therapeutics. 290 (2): 901–907. doi:10.1016/S0022-3565(24)34979-1. PMID 10411607.
- Aggarwal VK, Emme I, Fulford SY (February 2003). “Correlation between pK(a) and reactivity of quinuclidine-based catalysts in the Baylis-Hillman reaction: discovery of quinuclidine as optimum catalyst leading to substantial enhancement of scope”. The Journal of Organic Chemistry. 68 (3): 692–700. doi:10.1021/jo026671s. PMID 12558387.
External links
- Clinical trial number (NCT05656027 for “Phase 3 Evaluation of the Safety and Efficacy of LNZ101 for the Treatment of Presbyopia (CLARITY)” at ClinicalTrials.gov
- Clinical trial number (NCT05728944 for “Phase 3 Efficacy Study of LNZ101 for the Treatment of Presbyopia (CLARITY)” at ClinicalTrials.gov
- Clinical trial number (NCT05753189 for “Phase 3 Safety Study for the Treatment of Presbyopia Subjects” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Other names | LNZ101 |
| AHFS/Drugs.com | Vizz |
| License data | US DailyMed: Aceclidine |
| Routes of administration | Topical (ophthalmic solution) |
| ATC code | S01EB08 (WHO) |
| Legal status | |
| Legal status | US: ℞-only[1]In general: ℞ (Prescription only) |
| Identifiers | |
| IUPAC name | |
| CAS Number | 827-61-2 6109-70-2 |
| PubChem CID | 1979 |
| ChemSpider | 1902 |
| UNII | 0578K3ELIO |
| KEGG | D02750 |
| ChEMBL | ChEMBL20835 |
| CompTox Dashboard (EPA) | DTXSID2045658 |
| ECHA InfoCard | 100.011.431 |
| Chemical and physical data | |
| Formula | C9H15NO2 |
| Molar mass | 169.224 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
Zhou Y, Zhang Y, Zhao D, Yu X, Shen X, Zhou Y, Wang S, Qiu Y, Chen Y, Zhu F: TTD: Therapeutic Target Database describing target druggability information. Nucleic Acids Res. 2024 Jan 5;52(D1):D1465-D1477. doi: 10.1093/nar/gkad751. [Article]
///////////Aceclidine, APPROVALS 2025, FDA 2025, Vizz. To treat presbyopia, 827-61-2, 3-Acetoxyquinuclidine, 3-Quinuclidinol acetate (ester), Aceclidina, 0578K3ELIO, Glaucostat














