Brezivaptan
CAS 1370444-22-6
ANC-501, THY-1773, TS-121, 575OB1CKN0
MF C25H30ClN5O3 MW 484.0 g/mol
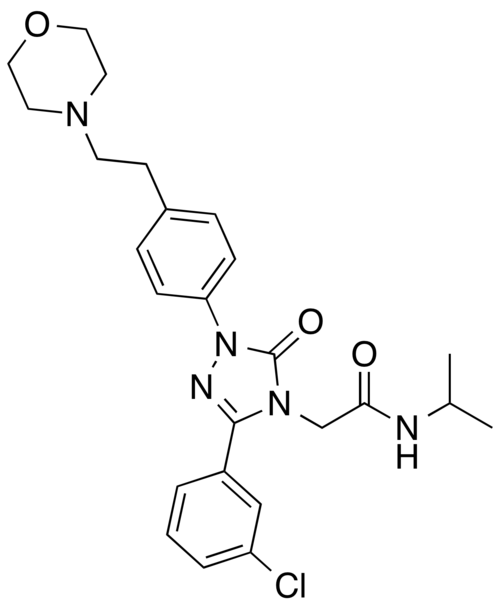
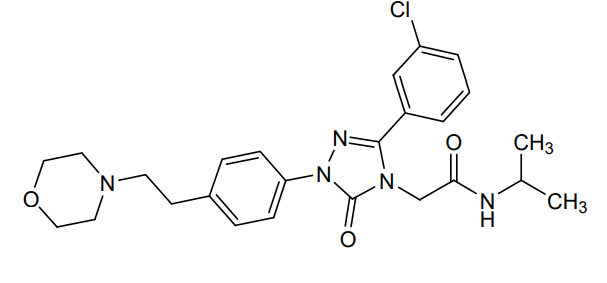
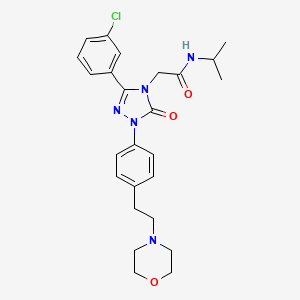
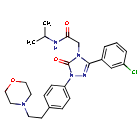
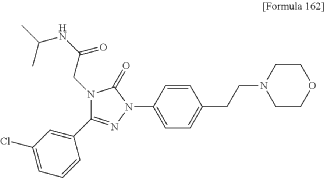
2-[3-(3-chlorophenyl)-1-{4-[2-(morpholin-4-yl)ethyl]phenyl}-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-N-(propan-2-yl)acetamide
2-[3-(3-chlorophenyl)-1-[4-(2-morpholin-4-ylethyl)phenyl]-5-oxo-1,2,4-triazol-4-yl]-N-propan-2-ylacetamide
vasopressin receptor antagonist
- ANC-501 in the Treatment of Adults With Major Depressive DisorderCTID: NCT05439603Phase: Phase 2Status: CompletedDate: 2024-12-31
- A Study to Evaluate the Safety and Efficacy of TS-121 as an Adjunctive Treatment for Major Depressive DisorderCTID: NCT03093025Phase: Phase 2Status: TerminatedDate: 2020-07-14
- Exploratory Study Using Positron Emission Tomography With TS-121 and [11C]TASP0410699 in Healthy Adult Male SubjectsCTID: NCT02448212Phase: Phase 1Status: CompletedDate: 2017-02-14
Brezivaptan[1] (developmental code names ANC-501, THY-1773, TS-121) is an orally active, selective vasopressin V1B receptor antagonist which is under development by Taisho Pharmaceutical for the adjunctive treatment of major depressive disorder.[2][3][4] As of November 2022, it is in phase II clinical trials for this indication.[2][3][5]
ANC-501 is under investigation in clinical trial NCT05439603 (ANC-501 in the Treatment of Adults With Major Depressive Disorder).
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US90328697&_cid=P11-MFYT6K-98384-1
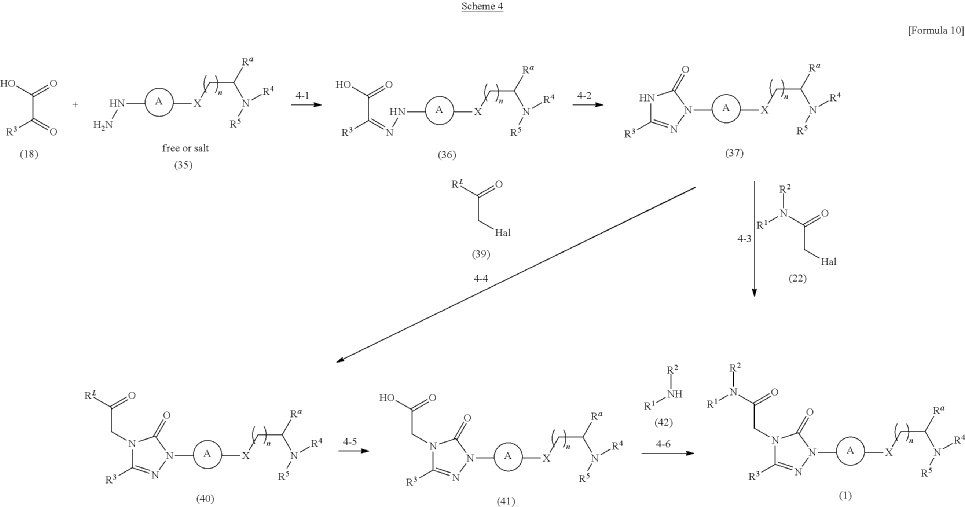
Synthesis of Example Aa-1
2-[3-(3-Chlorophenyl)-1-{4-[2-(morpholin-4-yl)ethyl]phenyl}-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-N-(propan-2-yl)acetamide
| A mixture of the compound (100 mg) prepared in Reference Example P-I1, morpholine (0.03 mL), N,N-diisopropylethylamine (0.35 mL), and MeCN (3.00 mL) was stirred at an outside temperature of 80° C. overnight. After cooling, the solvent was distilled off under reduced pressure. The residue was purified by column chromatography (SNAP Cartridge HP-Sil: 10 g, mobile phase: CHCl 3/MeOH=98/2 to 85/15 (v/v); and SNAP Cartridge KP-NH: 28 g, mobile phase: n-hexane/CHCl 3=80/20 to 0/100 (v/v)) and preparative thin-layer chromatography (PTLC) (1.0 mm silica gel 60F 254 plate, mobile phase: EtOAc/MeOH=95/5 (v/v)). The resulting crude product was washed with a solvent mixture of EtOAc and n-hexane (EtOAc/n-hexane=1/4 (v/v)) with stirring to yield the title compound (70 mg, colorless solid). |
PAT
- 1,2,4-triazolone derivativePublication Number: NZ-608729-APriority Date: 2010-10-01
- 1, 2, 4-triazolone derivativePublication Number: US-2013197217-A1Priority Date: 2010-10-01
- 1, 2, 4-triazolone derivative and use thereof as an antagonist on the arginine-vasopressin 1B receptorPublication Number: US-9193695-B2Priority Date: 2010-10-01Grant Date: 2015-11-24
- 1,2,4-triazolone derivative, substance and pharmaceutical compositionPublication Number: BR-112013007389-B1Priority Date: 2010-10-01
- 1,2,4-triazolone derivativePublication Number: EP-2623499-A1Priority Date: 2010-10-01
- 1,2,4-triazolone derivativePublication Number: EP-2623499-B1Priority Date: 2010-10-01Grant Date: 2015-04-22
- DERIVAT 1,2,4-TRIAZOLONAPublication Number: HR-P20150462-T1Priority Date: 2010-10-01
- 1,2,4-triazolone derivativePublication Number: HU-E025729-T2Priority Date: 2010-10-01
- 1,2,4-triazolone derivativePublication Number: IL-225091-APriority Date: 2010-10-01
- Methods of treating depression with 1,2,4-triazolone derivativesPublication Number: WO-2023235785-A1Priority Date: 2022-06-01
- 1,2,4-triazolone derivativePublication Number: AU-2011308403-A1Priority Date: 2010-10-01
- 1,2,4-triazolone derivativePublication Number: AU-2011308403-B2Priority Date: 2010-10-01Grant Date: 2014-08-21
- 1,2,4-Triazolone DerivativesPublication Number: CN-103119028-APriority Date: 2010-10-01
- 1,2,4-Triazolone DerivativesPublication Number: CN-103119028-BPriority Date: 2010-10-01Grant Date: 2016-05-25



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- PubChem. “Brezivaptan”. pubchem.ncbi.nlm.nih.gov. Retrieved 2024-08-15.
- “TS 121 -“. AdisInsight. Springer Nature Switzerland AG.
- “New Drug Pipeline – Taisho Pharmaceutical Holdings”.
- Kamiya M, Sabia HD, Marella J, Fava M, Nemeroff CB, Umeuchi H, Iijima M, Chaki S, Nishino I (September 2020). “Efficacy and safety of TS-121, a novel vasopressin V1B receptor antagonist, as adjunctive treatment for patients with major depressive disorder: A randomized, double-blind, placebo-controlled study”. Journal of Psychiatric Research. 128: 43–51. doi:10.1016/j.jpsychires.2020.05.017. PMID 32521250. S2CID 219587135.
- Inatani S, Mizuno-Yasuhira A, Kamiya M, Nishino I, Sabia HD, Endo H (May 2021). “Prediction of a clinically effective dose of THY1773, a novel V1B receptor antagonist, based on preclinical data”. Biopharmaceutics & Drug Disposition. 42 (5): 204–217. doi:10.1002/bdd.2273. PMC 8252455. PMID 33734452.
External links
- Clinical trial number NCT03093025 for “A Study to Evaluate the Safety and Efficacy of TS-121 as an Adjunctive Treatment for Major Depressive Disorder” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Other names | TS-121; TS121; TS-1211; TS1211; THY1773; THY-1773; ANC-501; ANC501 |
| Routes of administration | By mouth |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1370444-22-6 |
| PubChem CID | 56952080 |
| DrugBank | DB18907 |
| ChemSpider | 129325033 |
| UNII | 575OB1CKN0 |
| ChEMBL | ChEMBL5314910 |
| Chemical and physical data | |
| Formula | C25H30ClN5O3 |
| Molar mass | 484.00 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
////////////Brezivaptan, ANC-501, THY-1773, TS-121, ANC 501, THY 1773, TS 121, 575OB1CKN0














