Dapolsertib
CAS 1616359-00-2
MF C15H18Br2N4O MW 446.14 g/mol
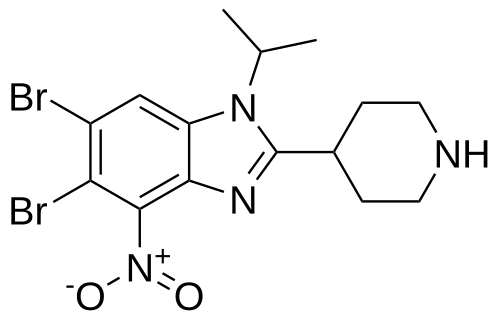
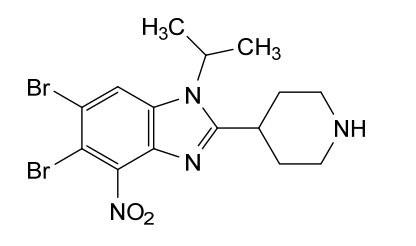
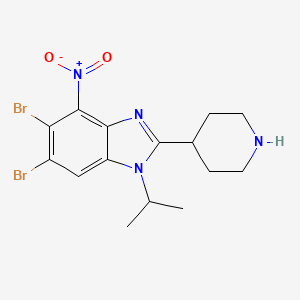
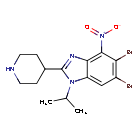
5,6-dibromo-4-nitro-2-piperidin-4-yl-1-propan-2-ylbenzimidazole
5,6-dibromo-4-nitro-2-(piperidin-4-yl)-1-(propan-2-yl)-1H-1,3-benzimidazole
serine/ threonine kinase inhibitor, antineoplastic
Ryvu Therapeutics SA, MEN1703, SEL24-B489
- SEL24-B489
- SEL-24 free base
- 9M7X64VTLI
- SEL-24
Dapolsertib is an investigational new drug that is being evaluated for the treatment of cancer. It is dual inhibitor of PIM family of serine/threonine protein kinases and mutant forms of FMS-related tyrosine kinase 3 (FLT3) that is being developed by Ryvu Therapeutics SA.[1]
Dapolsertib is an orally available inhibitor of PIM family serine/threonine protein kinases and mutant forms of FMS-related tyrosine kinase 3 (FLT3; STK1) with potential antineoplastic activity. Upon oral administration, dapolsertib binds to and inhibits the kinase activities of PIM-1, -2 and -3, and mutant forms of FLT3, which may result in the interruption of the G1/S phase cell cycle transition, an inhibition of cell proliferation, and an induction of apoptosis in tumor cells that overexpress PIMs or express mutant forms of FLT3. FLT3, a tyrosine kinase receptor that is overexpressed or mutated in various cancers, plays a role in signaling pathways that regulate hematopoietic progenitor cell proliferation, and in leukemic cell proliferation and survival. PIM kinases, downstream effectors of many cytokine and growth factor signaling pathways, including the FLT3 signaling pathway, play key roles in cell cycle progression and apoptosis inhibition and may be overexpressed in various malignancies.
- MEN1703 (SEL24) in Participants With Acute Myeloid LeukemiaCTID: NCT03008187Phase: Phase 1/Phase 2Status: CompletedDate: 2025-04-29
- MEN1703 (SEL24) to Treat Relapsed or Refractory Aggressive B-cell Non-Hodgkin Lymphoma (JASPIS-01)CTID: NCT06534437Phase: Phase 2Status: RecruitingDate: 2025-04-11
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014096388&_cid=P12-MG5YKY-59978-1
3.9. Compounds of Example 26:
3.9. Compounds of Example 26:
5,6-dibromo-4-nitro-2-(piperidin-4-yl)-1-(propan-2-yl)-1H-1,3-benzodiazole (Example 26A):
4,5-dibromo-1-N-(propan-2-yl)benzene-1,2-diamine (2,8g, 9,lmmol) and
isonipeconic acid (1,17g, 9,lmmol) were taken up in phosphoric acid (17,82g, 0,18mol). The resulting mixture was stirred at 180°C for 3,5 hours. The mixture was allowed to cool to RT and diluted with water to 200ml. The solution was basified to pH 14.0 using solid NaOH. The resulting precipitate was then filtered off and washed repeatedly with MeOH. The filtrate was concentrated in-vacuo. The product was purified on Al2O3 (basic) using DCM/MeOH/NH3 sat. in MEOH (25: 15: 1). The obtained product (8,7mmol, 3,9g) was dissolved in cone. H2SO4 (30ml). Next KNO3 (8,7mmol, 0,89g) was added in one portion at 0° C. The resulting mixture was stirred at 0°C for 3h and at RT overnight. Then the mixture was poured onto ice. The product was filtered and washed with water.The product was purified on on Al2O3 (basic) using DCM/MeOH/NH3 sat. in MEOH (25: 15: 1) to afford 5,6-dibromo-4- nitro-2-(piperidin-4-yl)-1-(propan-2-yl)-1H-1,3-benzodiazole (1,9g). 1H NMR (600 MHz, DMSO) δ 8.74 (bs, 1H), 8.48 (s, 1H), 8.35 (bs, 1H), 4.94 (hept, J = 6.8 Hz, 1H), 3.52 – 3.46 (m, 1H), 3.42 – 3.37 (m, 2H), 3.08 (bs, 2H), 2.07 – 1.96 (m, 4H), 1.60 (d, J = 6.9 Hz, 6H). m/z 446,8; rt 2,7min.
5,6-dibromo-4-nitro-2-(piperidin-4-yl)-1-(propan-2-yl)-1H-1,3-benzodiazole (Example 26A):
4,5-dibromo-1-N-(propan-2-yl)benzene-1,2-diamine (2,8g, 9,lmmol) and
isonipeconic acid (1,17g, 9,lmmol) were taken up in phosphoric acid (17,82g, 0,18mol). The resulting mixture was stirred at 180°C for 3,5 hours. The mixture was allowed to cool to RT and diluted with water to 200ml. The solution was basified to pH 14.0 using solid NaOH. The resulting precipitate was then filtered off and washed repeatedly with MeOH. The filtrate was concentrated in-vacuo. The product was purified on Al2O3 (basic) using DCM/MeOH/NH3 sat. in MEOH (25: 15: 1). The obtained product (8,7mmol, 3,9g) was dissolved in cone. H2SO4 (30ml). Next KNO3 (8,7mmol, 0,89g) was added in one portion at 0° C. The resulting mixture was stirred at 0°C for 3h and at RT overnight. Then the mixture was poured onto ice. The product was filtered and washed with water.The product was purified on on Al2O3 (basic) using DCM/MeOH/NH3 sat. in MEOH (25: 15: 1) to afford 5,6-dibromo-4- nitro-2-(piperidin-4-yl)-1-(propan-2-yl)-1H-1,3-benzodiazole (1,9g). 1H NMR (600 MHz, DMSO) δ 8.74 (bs, 1H), 8.48 (s, 1H), 8.35 (bs, 1H), 4.94 (hept, J = 6.8 Hz, 1H), 3.52 – 3.46 (m, 1H), 3.42 – 3.37 (m, 2H), 3.08 (bs, 2H), 2.07 – 1.96 (m, 4H), 1.60 (d, J = 6.9 Hz, 6H). m/z 446,8; rt 2,7min.
PAT
Novel benzimidazole derivatives as kinase inhibitors
Publication Number: WO-2014096388-A2
Priority Date: 2012-12-21
- Novel benzimidazole derivatives as kinase inhibitorsPublication Number: KR-20150095908-APriority Date: 2012-12-21
- Benzimidazole derivatives as kinase inhibitorsPublication Number: US-10174013-B2Priority Date: 2012-12-21Grant Date: 2019-01-08
- Novel Benzimidazole Derivatives as Kinase InhibitorsPublication Number: US-2015336967-A1Priority Date: 2012-12-21
- Novel benzimidazole derivatives as kinase inhibitorsPublication Number: US-2017152249-A1Priority Date: 2012-12-21
- Benzimidazole derivatives as Kinase InhibitorsPublication Number: US-9388192-B2Priority Date: 2012-12-21Grant Date: 2016-07-12
- Novel benzimidazole derivatives as kinase inhibitorsPublication Number: EP-2935244-B1Priority Date: 2012-12-21Grant Date: 2018-06-27
- Novel benzimidazole derivatives as kinase inhibitorsPublication Number: ES-2688395-T3Priority Date: 2012-12-21Grant Date: 2018-11-02
- Novel benzimidazole derivatives as kinase inhibitorsPublication Number: JP-2016503779-APriority Date: 2012-12-21
- Novel benzimidazole derivatives as kinase inhibitorsPublication Number: JP-6169185-B2Priority Date: 2012-12-21Grant Date: 2017-07-26
- Novel benzimidazole derivatives as kinase inhibitorsPublication Number: KR-101779272-B1Priority Date: 2012-12-21Grant Date: 2017-09-18



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | MEN1703, SEL24-B489 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1616359-00-2 |
| PubChem CID | 76286825 |
| IUPHAR/BPS | 13204 |
| ChemSpider | 81367232 |
| UNII | 9M7X64VTLI |
| ChEMBL | ChEMBL4467168 |
| Chemical and physical data | |
| Formula | C15H18Br2N4O2 |
| Molar mass | 446.143 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- Wu M, Li C, Zhu X (December 2018). “FLT3 inhibitors in acute myeloid leukemia”. Journal of Hematology & Oncology. 11 (1) 133. doi:10.1186/s13045-018-0675-4. PMC 6280371. PMID 30514344.
//////////Dapolsertib, antineoplastic, MEN1703, SEL24-B489, MEN 1703, SEL24 B489, Ryvu Therapeutics SA














