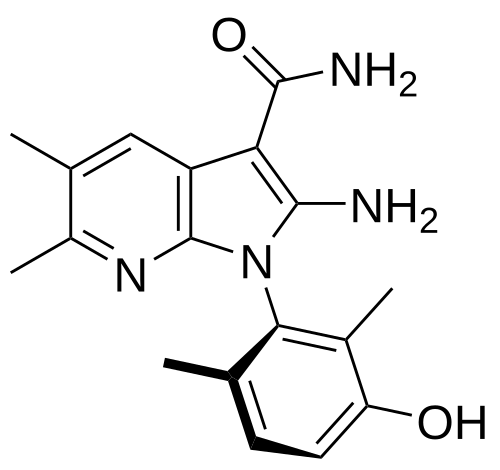
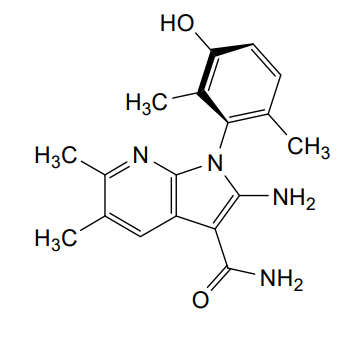
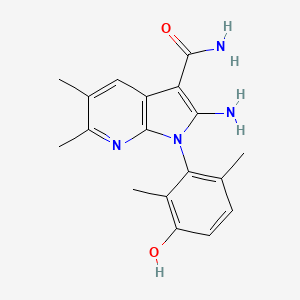
Lunresertib
CAS 2719793-90-3
MF C18H20N4O2 MW 324.4 g/mol
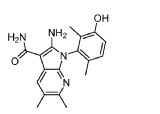
(1P)-2-amino-1-(3-hydroxy-2,6-dimethylphenyl)-5,6-dimethyl1H-pyrrolo[2,3-b]pyridine-3-carboxamide
serine/ threonine kinase inhibitor, antineoplastic, N95U3A7N57, RP-6306, RP 6306
2-Amino-1-(3-hydroxy-2,6-dimethylphenyl)-5,6-dimethylpyrrolo[2,3-b]pyridine-3-carboxamide
Lunresertib is an investigational new drug that is being evaluated for the treatment of cancer. It is an oral small molecule inhibitor of PKMYT1, developed by Repare Therapeutics.[1] This drug targets cell cycle regulation in tumors with specific genetic alterations, including CCNE1 amplifications or FBXW7 and PPP2R1A loss of function mutations. It is currently in phase 1/2 clinical trials, both as monotherapy or in combination with camonsertib, an ATR inhibitor.[2]
Lunresertib is an orally bioavailable inhibitor of the human membrane-associated tyrosine– and threonine-specific cdc2-inhibitory kinase (PKMYT1), with potential antineoplastic activity. Upon oral administration, lunresertib targets, binds to and inhibits the activity of PKMYT1. This results in the inhibition of CDK1 phosphorylation, which may promote both premature mitosis and a prolonged mitotic arrest, and lead to the accumulation of unrepaired DNA damage and apoptosis in susceptible tumor cells, such as CCNE1-overexpressing tumor cells. PKMYT1 phosphorylates CDK1 specifically when CDK1 is complexed to cyclins, which blocks progression from G2 into mitosis.NCI Thesaurus (NCIt)
- Study of RP-6306 With FOLFIRI in Advanced Solid TumorsCTID: NCT05147350Phase: Phase 1Status: TerminatedDate: 2025-08-20
- Study of RP-6306 Alone or in Combination With RP-3500 or Debio 0123 in Patients With Advanced Solid TumorsCTID: NCT04855656Phase: Phase 1Status: RecruitingDate: 2025-08-06
- RP-6306 in Patients With Advanced CancerCTID: NCT05605509Phase: Phase 2Status: Active, not recruitingDate: 2025-07-14
- Study of RP-6306 With Gemcitabine in Advanced Solid TumorsCTID: NCT05147272Phase: Phase 1Status: TerminatedDate: 2025-06-17
- Liquid-biopsy Informed Platform Trial to Evaluate CDK4/6-inhibitor Resistant ER+/HER2- Metastatic Breast CancerCTID: NCT05601440Phase: Phase 2Status: RecruitingDate: 2025-01-14
- Phase 1 Study of RP-6306 With Carboplatin and Paclitaxel in TP53 Ovarian and Uterine Cancer
- CTID: NCT06107868
- Phase: Phase 1
- Status: Active, not recruiting
- Date: 2024-03-22
PAT
- Compounds, Pharmaceutical Compositions, and Methods of Preparing and Using CompoundsPublication Number: JP-2023521633-APriority Date: 2020-04-01
- Compounds, pharmaceutical compositions, and methods of preparing compounds and of their usePublication Number: US-2023151014-A1Priority Date: 2020-04-01
- Methods of using myt1 inhibitorsPublication Number: US-2023158022-A1Priority Date: 2020-04-01
- Compounds, pharmaceutical compositions, and methods of preparing compounds and of their usePublication Number: EP-4126879-A1Priority Date: 2020-04-01
- Compounds, pharmaceutical compositions, and methods of preparing compounds and of their usePublication Number: IL-296934-APriority Date: 2020-04-01
- Compounds, pharmaceutical compositions, and methods of making the compounds and methods of using themPublication Number: KR-20230011279-APriority Date: 2020-04-01
- Compounds, pharmaceutical compositions and methods of making compounds and methods of their usePublication Number: CN-115916783-APriority Date: 2020-04-01
- Methods of using MYT1 inhibitorsPublication Number: JP-2023519430-APriority Date: 2020-04-01
- Methods of using myt1 inhibitorsPublication Number: WO-2021195782-A1Priority Date: 2020-04-01
- Compounds, pharmaceutical compositions, and methods of preparing compounds and of their usePublication Number: AU-2021250744-A1Priority Date: 2020-04-01
- Methods of using myt1 inhibitorsPublication Number: CA-3173955-A1Priority Date: 2020-04-01
- Methods of using MYT1 inhibitorsPublication Number: CN-115811976-APriority Date: 2020-04-01
- Methods of using myt1 inhibitorsPublication Number: EP-4125907-A1Priority Date: 2020-04-01
SYN
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021195781&_cid=P20-MHLE6P-37080-1
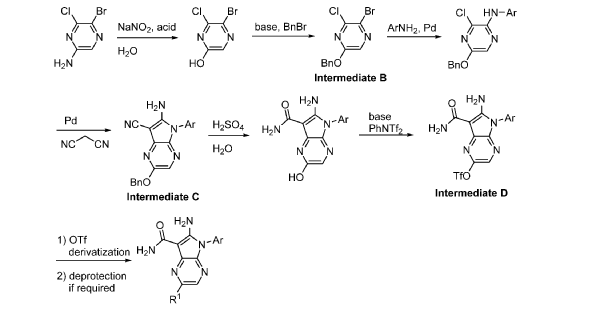
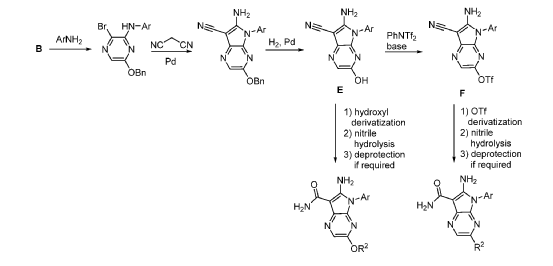
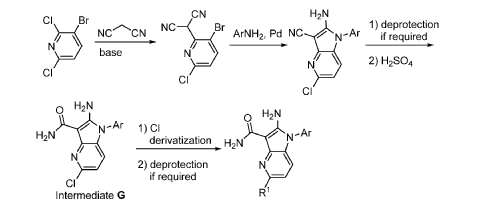
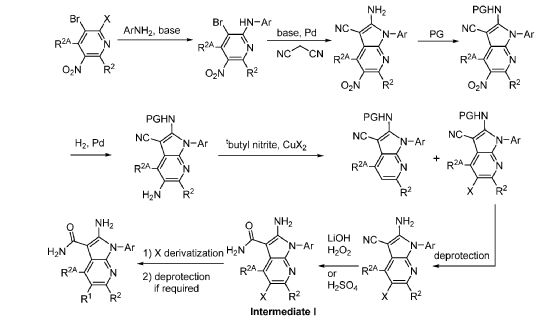
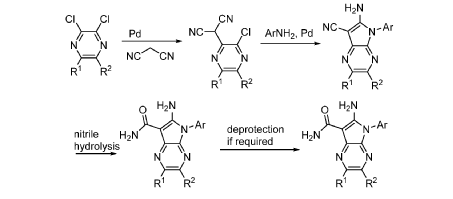
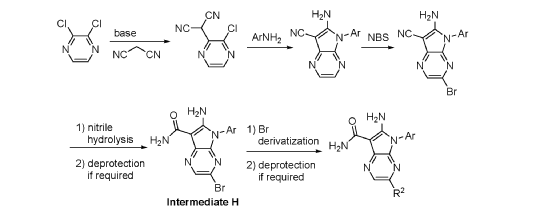
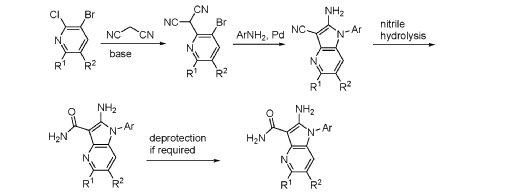
Step 9. To a suspension of 2-amino-1-(3-methoxy-2,6-dimethyl-phenyl)-5,6-dimethyl-pyrrolo[2,3-b]pyridine-3-carboxamide (2.22 g, 6.56 mmol, 77% purity) in DCM (25 mL) was added tribromoborane in DCM (1 M, 26 mmol, 26 mL) dropwise. The reaction mixture was stirred at RT for 45 min, then concentrated to dryness. The crude product was taken in DCM and placed in an ice bath and MeOH was added carefully (exotherm). The mixture was concentrated to dryness then co-evaporated twice with MeOH. The residue was triturated with saturated aqueous NaHCO3. The solids were collected by filtration on a Buchner funnel, washed with H2O and air-dried. The still wet solid was dissolved in DCM/MeOH, concentrated to dryness and triturated in 20% MeOH/DCM (50 mL). The solid was collected by filtration, washed with 20% MeOH/DCM, air-dried then dried in vacuo to afford 2-amino-1-(3-hydroxy-2,6-dimethyl-phenyl)-5,6-dimethyl-pyrrolo[2,3-b]pyridine-3-carboxamide (1.60g, 75% yield) as a light beige solid. MS: [M+1]: 325.1. A different batch was purified by preparative HPLC to yield 2-amino-1-(3-hydroxy-2,6-dimethyl-phenyl)-5,6-dimethyl-pyrrolo[2,3-b]pyridine-3-carboxamide (63% yield) as an off-white fluffy solid.
1H NMR (400 MHz, DMSO-d6) δ 9.51 (s, 1H), 7.82 (s, 1H), 7.05 (d, J = 8.3 Hz, 1H), 6.90 (d, J =
8.2 Hz, 1H), 6.71 (br s, 2H), 6.64 (br s, 2H), 2.26 (s, 3H), 2.23 (s, 3H), 1.74 (s, 3H), 1.65 (s, 3H). MS: [M+1]: 325.1.
Chiral SFC separation of Compound 181 (1.60g, 4.93 mmol) (Instrument: Waters Prep 100 SFC-MS; Column: Phenomenex Lux Cellulose-2, 30 x 250 mm, 5 μm; Conditions: isocratic at 55% IPA + 10mM Ammonium Formate with 45% CO2 ; Flow Rate: 70 mL/min) provided
Compound 182 and Compound 183.
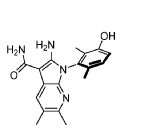
Compound 182 from SFC separation of 181. Peak 1 (retention time 3.94 min, 99.86%): (S)-2- amino-1-(3-hydroxy-2,6-dimethyl-phenyl)-5,6-dimethyl-pyrrolo[2,3-b]pyridine-3-carboxamide (381 mg) was obtained as an off white fluffy solid. 1H NMR (400 MHz, DMSO-d6) δ 9.50 (s, 1H), 7.83 (s, 1 H), 7.05 (d, J = 8.3 Hz, 1H), 6.90 (d, J = 8.3 Hz, 1H), 6.72 (s, 2H), 6.65 (s, 2H), 2.26 (s, 3H), 2.24 (s, 3H), 1.74 (s, 3H), 1.65 (s, 3H). MS: [M+1]: 325.1.
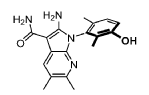
Compound 183 from SFC separation of 181. Peak 2 (retention time 4.35 min, 98.09%): (R)-2- amino-1-(3-hydroxy-2,6-dimethyl-phenyl)-5,6-dimethyl-pyrrolo[2,3-b]pyridine-3-carboxamide (495 mg) was obtained as an off white fluffy solid. 1H NMR (400 MHz, DMSO-d6) δ 9.50 (s, 1H), 7.83 (s, 1 H), 7.05 (d, J = 8.2 Hz, 1H), 6.90 (d, J = 8.2 Hz, 1H), 6.72 (s, 2H), 6.66 (s, 2H), 2.26 (s, 3H), 2.24 (s, 3H), 1.74 (s, 3H), 1.65 (s, 3H). MS: [M+1]: 325.1.
SYN
https://pubs.acs.org/doi/full/10.1021/acs.oprd.4c00493
REF
- The Science and Art of Structure-Based Virtual ScreeningPublication Name: ACS Medicinal Chemistry LettersPublication Date: 2024-03-25PMCID: PMC11017385PMID: 38628791DOI: 10.1021/acsmedchemlett.4c00093
- Discovery of an Orally Bioavailable and Selective PKMYT1 Inhibitor, RP-6306Publication Name: Journal of Medicinal ChemistryPublication Date: 2022-07-26PMCID: PMC9837800PMID: 35880755DOI: 10.1021/acs.jmedchem.2c00552
- CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibitionPublication Name: NaturePublication Date: 2022-04-20PMCID: PMC9046089PMID: 35444283DOI: 10.1038/s41586-022-04638-9
- Contributions in the domain of cancer research: Review¶Negative regulators of cyclin-dependent kinases and their roles in cancersPublication Name: Cellular and molecular life sciences : CMLSPublication Date: 2001-11PMCID: PMC11337304PMID: 11766887DOI: 10.1007/pl00000826



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | RP-6306 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2719793-90-3 |
| PubChem CID | 156869388 |
| ChemSpider | 115008046 |
| UNII | N95U3A7N57 |
| KEGG | D12736 |
| ChEMBL | ChEMBL5199076 |
| Chemical and physical data | |
| Formula | C18H20N4O2 |
| Molar mass | 324.384 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- Szychowski J, Papp R, Dietrich E, Liu B, Vallée F, Leclaire ME, et al. (August 2022). “Discovery of an Orally Bioavailable and Selective PKMYT1 Inhibitor, RP-6306”. Journal of Medicinal Chemistry. 65 (15): 10251–10284. doi:10.1021/acs.jmedchem.2c00552. PMC 9837800. PMID 35880755.
- Previtali V, Bagnolini G, Ciamarone A, Ferrandi G, Rinaldi F, Myers SH, et al. (July 2024). “New Horizons of Synthetic Lethality in Cancer: Current Development and Future Perspectives”. Journal of Medicinal Chemistry. 67 (14): 11488–11521. doi:10.1021/acs.jmedchem.4c00113. PMC 11284803. PMID 38955347.
///////lunresertib, Serine/ threonine kinase inhibitor, antineoplastic, N95U3A7N57, RP-6306, RP 6306














