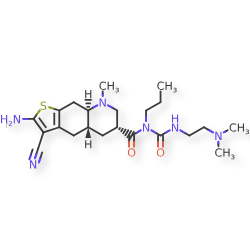
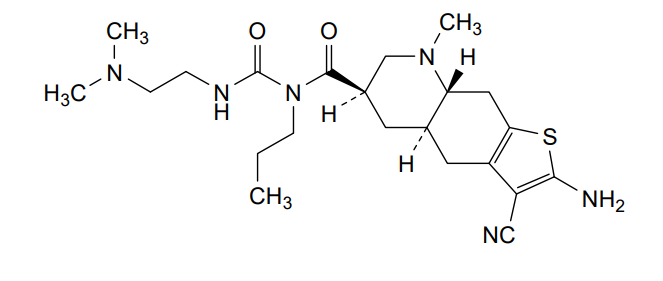
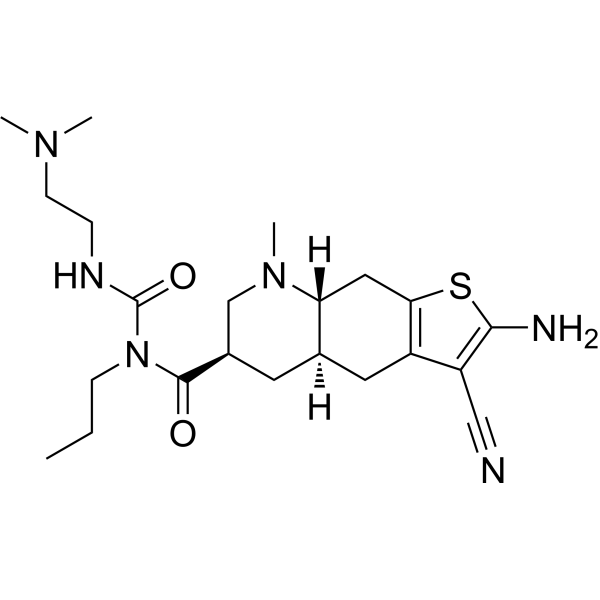

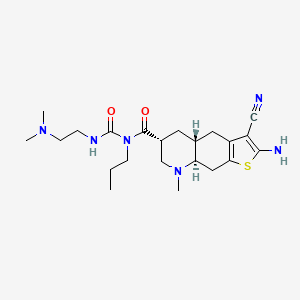
Matsupexole
CAS 1399442-97-7
MF C22H34N6O2S, Molecular Weight, 446.61
(4aR,6R,8aR)-2-amino-3-cyano-N-{[2-(dimethylamino)ethyl]carbamoyl}-8-methyl-N-propyl 4,4a,5,6,7,8,8a,9-octahydrothieno[3,2-g]quinoline-6-carboxamide
(4aR,6R,8aR)-2-amino-3-cyano-N-[2-(dimethylamino)ethylcarbamoyl]-8-methyl-N-propyl-4a,5,6,7,8a,9-hexahydro-4H-thieno[3,2-g]quinoline-6-carboxamide
dopamine receptor agonist, Phase 2, Parkinson’s disease, K4UEG65HTX
- OriginatorKissei Pharmaceutical
- DeveloperAffaMed Therapeutics; Kissei Pharmaceutical
- ClassAmides; Amines; Antiparkinsonians; Dimethylamines; Ethylenediamines; Nitriles; Quinolines; Small molecules; Thiophenes; Urea compounds
- Mechanism of ActionDopamine receptor agonists
- Phase IIParkinson’s disease
- 28 Aug 2025Chemical structure information added.
- 06 Sep 2021Kissei Pharmaceutical completes a phase II trial in Parkinson’s disease (In adults, In elderly) in Japan (PO) (NCT04867551)
- 04 Aug 2021Phase-II clinical trials in Parkinson’s disease in China (PO) (Kissei Pharmaceutical pipeline, August 2021)
PAT
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: WO-2012124649-A1Priority Date: 2011-03-14
- COMPOUNDS DERIVED FROM OCTAIDROTHIENOQUINOLINE, PHARMACEUTICAL COMPOSITION AND PHARMACEUTICAL AGENT COMPRISING SUCH COMPOUNDSPublication Number: BR-112013023575-B1Priority Date: 2011-03-14
- New octahydrothienoquinoline derivative, pharmaceutical composition containing derivative, and using themPublication Number: RU-2573399-C2Priority Date: 2011-03-14Grant Date: 2016-01-20
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: SG-193400-A1Priority Date: 2011-03-14
- Novel octahydrothienoquinoline derivatives, pharmaceutical compositions containing the same, and their usesPublication Number: TW-I537274-BPriority Date: 2011-03-14Grant Date: 2016-06-11
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: US-2014243311-A1Priority Date: 2011-03-14
- Octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: US-9138434-B2Priority Date: 2011-03-14Grant Date: 2015-09-22
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: HU-E033449-T2Priority Date: 2011-03-14
- Novel octahydrothienoquinoline derivatives, pharmaceutical compositions containing them and their usePublication Number: JP-5563716-B2Priority Date: 2011-03-14Grant Date: 2014-07-30
- Novel octahydrothienoquinoline derivatives, pharmaceutical compositions containing them and their usePublication Number: JP-WO2012124649-A1Priority Date: 2011-03-14
- Novel octahydrothienoquinoline derivatives, pharmaceutical compositions containing them and uses thereofPublication Number: KR-20140010137-APriority Date: 2011-03-14
- NEW OCTAHYDROTHYENOCHINOLINE DERIVATIVE, PHARMACEUTICAL COMPOSITION CONTAINING A DERIVATIVE AND THEIR APPLICATIONPublication Number: RU-2013145799-APriority Date: 2011-03-14
- Novel octahydrothienoquinoline derivatives, pharmaceutical compositions comprising said derivatives and their usesPublication Number: CN-103443106-BPriority Date: 2011-03-14Grant Date: 2015-09-30
- New octahydrothienoquinoline derivative, pharmaceutical composition comprising the derivative, and use thereofPublication Number: DK-2687532-T3Priority Date: 2011-03-14Grant Date: 2017-02-20
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: EP-2687532-A1Priority Date: 2011-03-14
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: EP-2687532-B1Priority Date: 2011-03-14Grant Date: 2016-12-14
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising the derivative, and use thereofPublication Number: ES-2613658-T3Priority Date: 2011-03-14Grant Date: 2017-05-25
- Novel dopamine D2 receptor agonistPublication Number: JP-2014074013-APriority Date: 2012-09-12
- Novel dopamine D2 receptor agonistPublication Number: JP-6177061-B2Priority Date: 2012-09-12Grant Date: 2017-08-09
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: AU-2012227428-A1Priority Date: 2011-03-14
- Novel octahydrothienoquinoline derivative, pharmaceutical composition comprising derivative, and use of thesePublication Number: AU-2012227428-B2Priority Date: 2011-03-14Grant Date: 2016-05-05
- Novel octahydrothienoquinoline derivatives, pharmaceutical compositions comprising said derivatives and their usesPublication Number: CN-103443106-APriority Date: 2011-03-14
- Succinate of octahydrothienoquinoline compound, and crystals thereofPublication Number: WO-2022009815-A1Priority Date: 2020-07-06
- Succinate of octahydrothienoquinoline compound and its crystalPublication Number: CN-115803329-APriority Date: 2020-07-06
- Succinate salts of octahydrothienoquinoline compounds and crystals thereofPublication Number: KR-20230035050-APriority Date: 2020-07-06
- Succinate of octahydrothienoquinoline compound, and crystals thereofPublication Number: EP-4177257-A1Priority Date: 2020-07-06
- Succinate salts of octahydrothienoquinoline compound and crystals thereofPublication Number: US-2023286998-A1Priority Date: 2020-07-06
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022009815&_cid=P22-MHO952-66657-1
[0018]Example 11-{[(4aR,6R,8aR)-2-amino-3-cyano-8-methyl-4,4a,5,6,7,8,8a,9-octahydrothieno[3,2-g]quinolin-6-yl]carbonyl}-3-[2-(dimethylamino)ethyl]-1-propylurea sesquisuccinate monohydrate (Form I crystals of salt (A-1)) 102.8 g of acetone was added to 1-{[(4aR,6R,8aR)-2-amino-3-cyano-8-methyl-4,4a,5,6,7,8,8a,9-octahydrothieno[3,2-g]quinolin-6-yl]carbonyl}-3-[2-(dimethylamino)ethyl]-1-propylurea (22.00 g), the mixture was suspended, and the suspension was heated and stirred at an external temperature of 52°C to dissolve the suspension. Activated carbon (2.2 g) was added to this solution and stirred for 10 minutes. This suspension was hot filtered and washed with 35.2 g of acetone. 220.0 g of acetone was then added, and the reaction solution was heated to an external temperature of 52°C and stirred. Next, 44.0 g of water was added to the reaction solution. Separately, 8.73 g of succinic acid was dissolved in a mixed solution of 156.1 g of acetone and 19.8 g of water. This succinic acid solution was added dropwise to the reaction solution over approximately 10 minutes. The dropping funnel was washed with a mixed solution of 17.4 g of acetone and 2.2 g of water and then added dropwise to the reaction solution. The reaction solution was stirred at an internal temperature of 50°C for 1 hour and cooled to 15°C over 30 minutes. The reaction solution was stirred at an external temperature of 10°C for 2 hours, and the crystals were collected by filtration. The crystals were washed twice with 52.8 g of acetone. The obtained wet crystals were dried under reduced pressure at 50°C for 37 hours and then returned to room temperature under reduced pressure over 3 hours. The crystals were stored under air for 24 hours to obtain crystals (27.75 g) of the title compound.
1 H-NMR (DMSO-d6) (δ (ppm)): 0.85 (3H, t, J = 7.4Hz), 1.32 (1H, ddd, J=12.2Hz, 12.2Hz, 12.2Hz), 1.42-1.57 (2H, m), 1.57-1.70 (1H, m ), 1.89-2.00 (2H, m), 2.20-2.13 (1H, m), 2.13-2.28 (2H, m), 2.21 (3H, s), 2.24 (6H, s ), 2.35-2.48 (1H, m), 2.40 (6H, s), 2.46 (2H, t, J = 6.4Hz), 2.81-2.96 (2H, m), 3.00-3 .12 (1H, m), 3.21-3.33 (2H, m), 3.47-3.66 (2H, m), 6.99 (2H, s), 8.50-8.90 (1H, br).
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012124649&_cid=P22-MHO8UB-55660-1
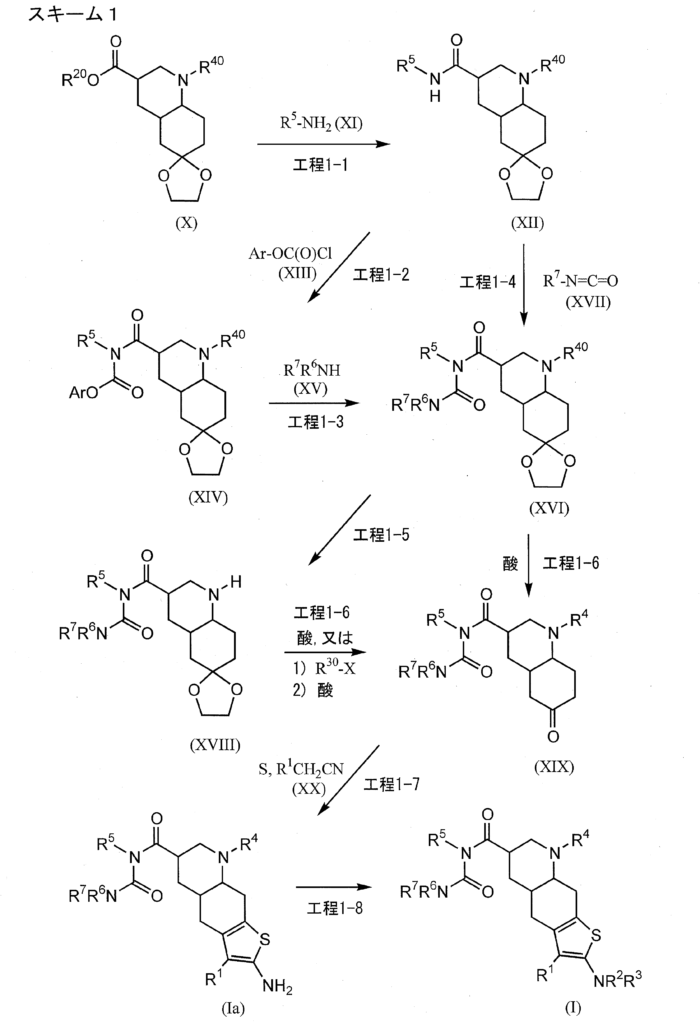
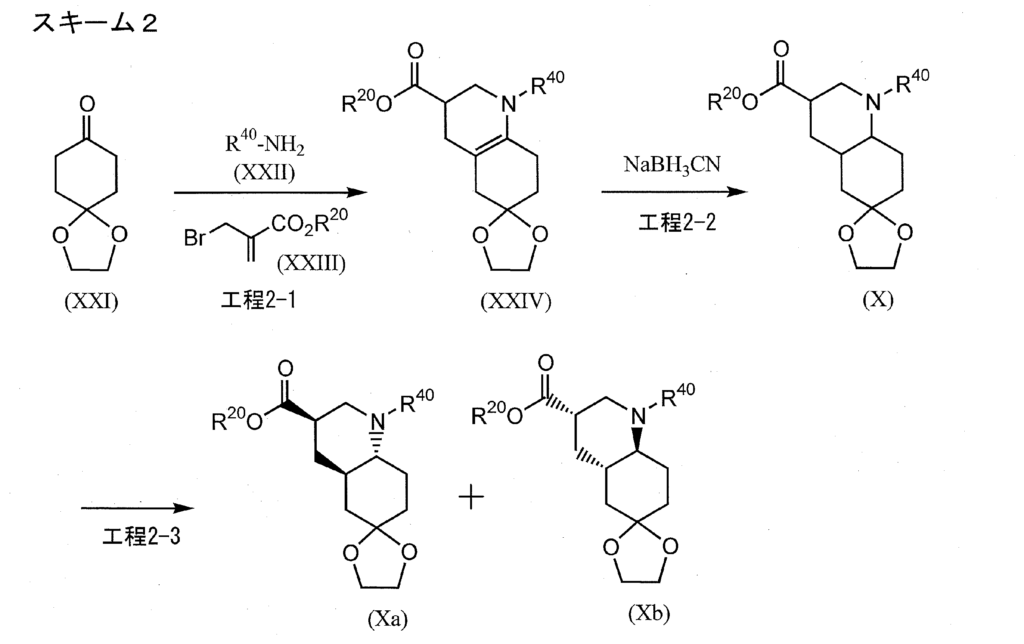
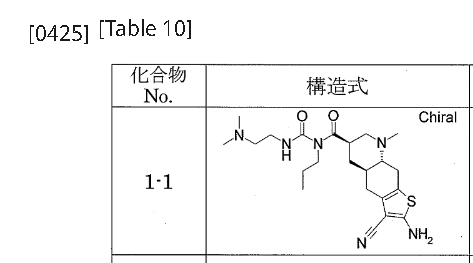
[0422]Example 1-11-{[(4aR,6R,8aR)-2-amino-3-cyano-8-methyl-4H,4aH,5H,6H,7H,8H,8aH,9H-thieno[3,2-g]quinolin-6-yl]carbonyl}-3-[2-(dimethylamino)ethyl]-1-propylurea (Compound 1-1) To a mixture of 1-{[(3R,4aR,8aR)-1-methyl-6-oxodecahydroquinolin-3-yl]carbonyl}-3-[2-(dimethylamino)ethyl]-1-propylurea (Reference Example 10-1) (1.602 g) and ethanol (44 mL) were added malononitrile (435 mg), morpholine (0.572 mL), and then elemental sulfur (282 mg) with stirring at room temperature, and the mixture was heated to 55°C and stirred for 1.5 hours. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure, and the residue was purified by column chromatography on aminopropyl silica gel (eluent: 0%-5% methanol/ethyl acetate, gradient elution) to give the title compound (1.479 g) as a solid.
1 H-NMR (CDCl
3 ) δ ppm: 0.94(3H, t, J=7.4Hz), 1.45-1.85(4H, m), 1.95-2.15(2H, m), 2.15-2.30(7H, m), 2.30-2.55(7H, m), 2.60-2.75(1H, m), 2.90-3.00(2H, m), 3.00-3.10(1H, m), 3.35-3.45(2H, m), 3.60-3.85(2H, m), 4.65(2H, s), 9.27(1H, br)[α]
D 29 =-105.54°(c=0.30, MeOH)
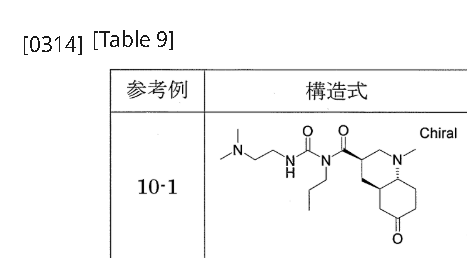
[0311]Reference Example 10-11-{[(3R,4aR,8aR)-1-methyl-6-oxodecahydroquinolin-3-yl]carbonyl}-3-[2-(dimethylamino)ethyl]-1-propylurea 1-{[(3’R,4’aR,8’aR)-1′-methyloctahydro-1’H-spiro[1,3-dioxolane-2,6′-quinoline]-3′-yl]carbonyl}-3-[2-(dimethylamino)ethyl]-1-propylurea (Reference Example 8-1) (2.366 g) was added to 2 mol/L hydrochloric acid (30 mL), and the mixture was stirred at room temperature for 2 hours. The reaction mixture was washed with diethyl ether, and then potassium carbonate was added to the aqueous layer to make it alkaline. The mixture was extracted with a methylene chloride/methanol mixed solvent (methylene chloride:methanol = 9:1). The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the title compound (1.605 g).
1 H-NMR (CDCl
3 ) δ ppm: 0.94 (3H, t, J=7.4 Hz), 1.45-1.90 (6H, m), 1.95-2.05 (1H, m), 2.10-2.55 (17H, m), 2.90-3.10 (2H, m), 3.30-3.45 (2H, m), 3.60-3.80 (2H, m), 9.22 (1H, brs).[α]
D 28 =-37.56° (c=0.38, MeOH).
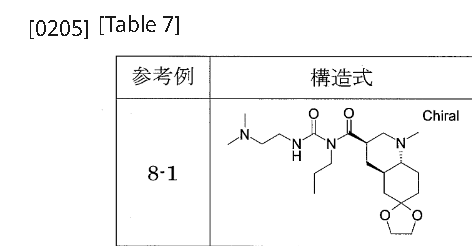
[0198]Reference Example 8-1To a mixture of phenyl 1-{[(3’R,4’aR,8’aR)-1′-methyloctahydro-1’H-spiro[1,3-dioxolane-2,6′-quinoline]-3′-yl]carbonyl}-3-[2-(dimethylamino)ethyl]-1-propylurea N-{[(3’R,4’aR,8’aR)-1′-methyloctahydro-1’H-spiro[1,3-dioxolane-2,6′-quinoline]-3′-yl]carbonyl}-N-propylcarbamate (Reference Example 6-1) (2.401 g) and 2-propanol (30 mL), N,N-dimethylethylenediamine (1.26 mL) was added with stirring at room temperature, and the mixture was heated to 53°C and stirred for 13 hours. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified by aminopropyl silica gel column chromatography (eluent: 0%-100% ethyl acetate/hexane, gradient elution) to give the title compound (2.383 g).
1 H-NMR (CDCl
3 ) δ ppm: 0.92(3H, t, J=7.4Hz), 1.35-1.50(3H, m), 1.50-1.90(8H, m), 2.00-2.15(1H, m), 2.26(6H, s), 2.31(3H, s), 2.37(1H, t, J=11.2Hz), 2.46(2H, t, J=6.4Hz), 2.85-3.10(2H, m), 3.35-3.45(2H, m), 3.60-3.70(1H, m), 3.70-3.80(1H, m), 3.90-4.00(4H, m), 9.33(1H, br)[α]
D 28 =-6.62°(c=0.31, MeOH)



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
///////matsupexole, dopamine receptor agonist, Phase 2, Parkinson’s disease, K4UEG65HTX














