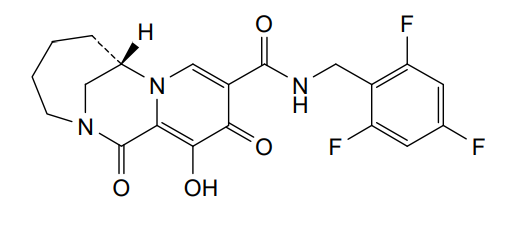

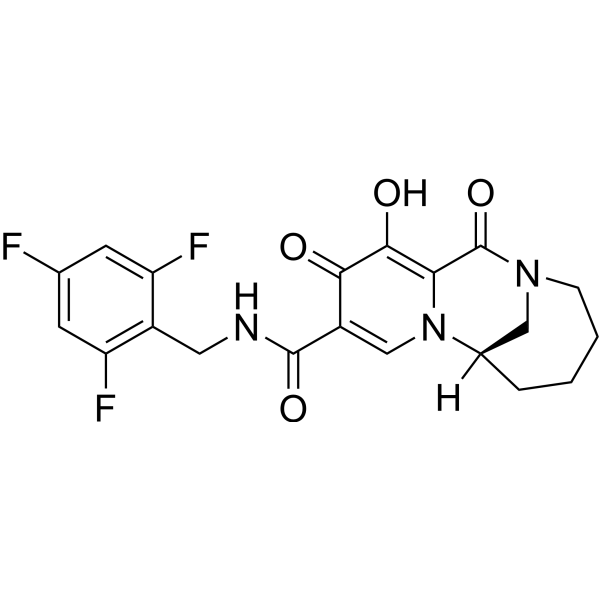
Odentegravir
CAS 2495436-99-0
MF C20H18F3N3O4 MW421.4 g/mol
(7S)-12-hydroxy-1,11-dioxo-N-[(2,4,6-trifluorophenyl)methyl]-1,4,5,6,7,11-hexahydro-3H-2,7-
methanopyrido [1,2-a][1,4]diazonine-10-carboxamide
(7S)-1,4,5,6,7,11-HEXAHYDRO-12-HYDROXY-1,11-DIOXO-N-((2,4,6-TRIFLUOROPHENYL)METHYL)-3H-2,7-METHANOPYRIDO(1,2-A)(1,4)DIAZONINE-10-CARBOXAMIDE
(7S)-12-HYDROXY-1,11-DIOXO-N-((2,4,6-TRIFLUOROPHENYL)METHYL)-1,4,5,6,7,11-HEXAHYDRO-3H-2,7-METHANOPYRIDO(1,2-A)(1,4)DIAZONINE-10-CARBOXAMIDE
3H-2,7-METHANOPYRIDO(1,2-A)(1,4)DIAZONINE-10-CARBOXAMIDE, 1,4,5,6,7,11-HEXAHYDRO-12-HYDROXY-1,11-DIOXO-N-((2,4,6-TRIFLUOROPHENYL)METHYL)-, (7S)-
antiviral, H8B26JZ4A4, orb2664247
Odentegravir is a small molecule drug classified as a
HIV integrase inhibitor, indicated by the “-tegravir” stem in its name. It is a chemical compound with the molecular formula
has been used in research for its antiviral properties.
- Drug Class: HIV integrase inhibitor
- Chemical Formula:
C20H18F3N3O4cap C sub 20 cap H sub 18 cap F sub 3 cap N sub 3 cap O sub 4𝐶20𝐻18𝐹3𝑁3𝑂4
- Molecular Weight:
421.12421.12421.12 Da (monoisotopic)
- Classification: Small molecule drug
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020197991&_cid=P12-MHY8KB-06018-1
Example 23: Preparation of racemic-12-hydroxy-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26), (7R)-12-hydroxy-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-
methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26-1) and (7S)-12-hydroxy-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26-2):

Synthesis of 12-Hydroxy-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26):
[0335] 12-(Benzyloxy)-1,11-dioxo-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxylic acid (57 mg, 0.155 mmol) was dissolved in DCM (2 mL) with (2,4,6-trifluorophenyl)methanamine (27 mg, 0.17 mmol) and triethylamine (60 mg, 0.464 mmol). HATU (60 mg, 0.186 mmol) was added and the mixture was stirred at room
temperature. After overnight reaction, the reaction was concentrated to dryness, purified by silicon gel chromatography to obtain compound 12-(benzyloxy)-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26a) MS (m/z) 512.06 [M+H]+.
[0336] Compound 12-(benzyloxy)-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26a) (7 mg, 0.014 mmol) was dissloved in Tolune (1 mL), then followed by the addition of TFA (1 mL). The resulting mixture was stirred at rt for overnight. The solvent was removed under vacuo an the residue was purifed by HPLC to obtain the title compound (26). MS (m/z) 422.091 [M+H]+.1H NMR (400 MHz, DMSO-d6) d 10.39 (t, J = 5.8 Hz, 1H), 8.45 (s, 1H), 7.24 – 7.11 (m, 2H), 4.72 (dd, J = 5.9, 2.9 Hz, 1H), 4.54 (dd, J = 6.0, 2.4 Hz, 2H), 4.11 (d, J = 13.3 Hz, 1H), 3.88 – 3.79 (m, 1H), 3.64 (dd, J = 14.7, 1.9 Hz, 1H), 3.05 (dq, J = 9.5, 3.4 Hz, 1H), 2.06 – 1.91 (m, 1H), 1.89 – 1.74 (m, 3H), 1.61 (d, J = 7.7 Hz, 1H), 1.11 (d, J = 12.7 Hz, 1H).
Synthesis of (7S)-12-hydroxy-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26-2) and (7R)-12-hydroxy-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26-1):
[0337] Racemic 12-(benzyloxy)-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26a) was separated by chiral HPLC separation (SFC chromatography on an IB 4.6X100mm 5mic column using MeOH(20) as co-solvent) to obtain compounds (7R)-12-(Benzyloxy)-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26a-1) and (7S)-12-(benzyloxy)-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26a-2)
[0338] Compound (7S)-12-(benzyloxy)-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26a-2) (20 mg, 0.039 mmol) was dissloved in Tolune (1 mL), then followed by the addition of TFA (1 mL). The resulting mixture was stireed at rt for overnight. The solvent was removed under vacuo an the residue was purifed by HPLC to obtain the title compound (26-2). (MS (m/z) 422.123 [M+H]+. 1H NMR (400 MHz, DMSO-d6) d 10.59 (s, 1H), 10.39 (d, J = 5.9 Hz, 1H), 8.45 (s, 1H), 7.18 (t, J = 8.6 Hz, 2H), 4.72 (s, 1H), 4.59 – 4.48 (m, 2H), 4.11 (d, J = 13.2 Hz, 1H), 3.85 (d, J = 14.6 Hz, 1H), 3.69 – 3.59 (m, 1H), 3.05 (ddd, J = 11.3, 6.7, 3.6 Hz, 1H), 1.97 (m, 1H), 1.87 – 1.71 (m, 3H), 1.67 – 1.55 (m, 1H), 1.10 (m, 1H).
[0339] Compound (7R)-12-(benzyloxy)-1,11-dioxo-N-(2,4,6-trifluorobenzyl)-1,4,5,6,7,11-hexahydro-3H-2,7-methanopyrido[1,2-a][1,4]diazonine-10-carboxamide (26a-1) ((20 mg, 0.039 mmol) was dissloved in Tolune (1 mL), then followed by the addition of TFA (1 mL). The resulting mixture was stireed at rt for overnight. The solvent was removed under vacuo an the residue was purifed by HPLC to obtain the title compound (26-1). MS (m/z) 422.116 [M+H]+. 1H NMR (400 MHz, DMSO-d6) d 10.58 (s, 1H), 10.39 (t, J = 5.8 Hz, 1H), 8.45 (s, 1H), 7.18 (dd, J = 9.2, 8.0 Hz, 2H), 4.73 (s, 1H), 4.58 – 4.49 (m, 2H), 4.11 (d, J = 13.3 Hz, 1H), 3.85 (d, J = 14.6 Hz, 1H), 3.65 (d, J = 14.2 Hz, 1H), 3.10 – 3.00 (m, 1H), 1.96 (m, 1H), 1.82 (d, J = 12.2 Hz, 3H), 1.61 (d, J = 7.4 Hz, 1H), 1.18 – 1.05 (m, 1H).
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023196875&_cid=P12-MHY8FJ-02517-1
PAT
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usesPublication Number: JP-2025013503-APriority Date: 2019-03-22
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usesPublication Number: KR-102714084-B1Priority Date: 2019-03-22Grant Date: 2024-10-08
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: KR-20240151256-APriority Date: 2019-03-22
- Bridged Tricyclic Carbamoylpyridone Compounds and Their Pharmaceutical UsePublication Number: ES-2927041-T3Priority Date: 2019-03-22Grant Date: 2022-11-03
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: US-11548902-B1Priority Date: 2019-03-22Grant Date: 2023-01-10
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: US-2023027019-A1Priority Date: 2019-03-22
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: AU-2020245350-B2Priority Date: 2019-03-22Grant Date: 2023-04-20
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: US-2023203061-A1Priority Date: 2019-03-22
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: US-11084832-B2Priority Date: 2019-03-22Grant Date: 2021-08-10
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: AU-2020245350-A1Priority Date: 2019-03-22
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: EP-3938047-A1Priority Date: 2019-03-22
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: EP-3938047-B1Priority Date: 2019-03-22Grant Date: 2022-06-22
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: EP-4122537-A1Priority Date: 2019-03-22
- Bridged tricyclic carbamoylpyridone compounds and uses thereofPublication Number: US-2023339971-A1Priority Date: 2022-04-06
- Bridged tricyclic carbamoylpyridone compounds and uses thereofPublication Number: US-2023339972-A1Priority Date: 2022-04-06
- Bridged tricyclic carbamoylpyridone compounds and uses thereofPublication Number: WO-2023196875-A1Priority Date: 2022-04-06
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: US-2020317689-A1Priority Date: 2019-03-22
- Bridged tricyclic carbamoylpyridone compounds and their pharmaceutical usePublication Number: WO-2020197991-A1Priority Date: 2019-03-22


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
//////Odentegravir, antiviral, H8B26JZ4A4, orb2664247














