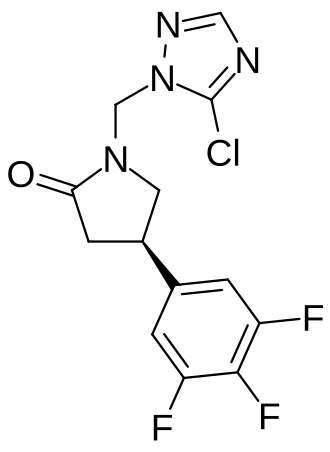
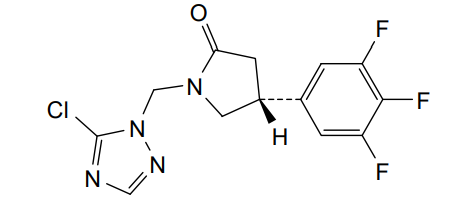

Plosaracetam
CAS 1651179-19-9
MF C13H10ClF3N4O MW330.69 g/mol
(4R)-1-[(5-chloro-1H-1,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one
(4R)-1-[(5-chloro-1,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one
(4R)-1-[(5-Chloro-1H-1,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluorophenyl)-2-pyrrolidinone
(4R)-1-[(5-chloro-1H-1,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one
2-Pyrrolidinone, 1-[(5-chloro-1H-1,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluorophenyl)-, (4R)-
synaptic vesicle glycoprotein 2A (SV2A) positive modulator, ABBV-552, ABBV552, SDI-118, SDI118, ABBV 552, ABBV552, SDI 118, SDI118, W3LYF2KQ6F
Plosaracetam (INNTooltip International Nonproprietary Name; developmental code names ABBV-552, SDI-118) is a synaptic vesicle glycoprotein 2A (SV2A) ligand which is under development for the treatment of Alzheimer’s disease and other cognition disorders.[1][3][4][2] In contrast to earlier SV2A ligands like levetiracetam and brivaracetam, polsaracetam does not have anticonvulsant activity and instead shows pro-cognitive effects.[2] The drug is being developed by UCB Biopharma and AbbVie.[1][3] As of October 2024, it is in phase 2 clinical trials for Alzheimer’s disease and phase 1 trials for cognition disorders.[1][3]
Plosaracetam is a small molecule drug. The usage of the INN stem ‘-racetam’ in the name indicates that Plosaracetam is a piracetam type amide type nootrope agent. Plosaracetam is under investigation in clinical trial NCT05199142 (A Study to Evaluate the Safety, Tolerability, and Pharmacodynamics of SDI-118 in Elderly Male and Female Study Participants With Cognitive Decline). Plosaracetam has a monoisotopic molecular weight of 330.05 Da.
PAT
- Compounds for Enhancing the Cognitive FunctionPublication Number: US-2016185761-A1Priority Date: 2013-08-02
- Compounds for enhancing the cognitive functionPublication Number: US-9630948-B2Priority Date: 2013-08-02Grant Date: 2017-04-25
- Compounds for enhancing the cognitive functionPublication Number: WO-2015014785-A1Priority Date: 2013-08-02
- Connections to improve cognitive functionPublication Number: DK-3027606-T3Priority Date: 2013-08-02Grant Date: 2018-04-30
- Compounds for enhancing the cognitive functionPublication Number: EP-3027606-A1Priority Date: 2013-08-02
- Compounds for enhancing the cognitive functionPublication Number: EP-3027606-B1Priority Date: 2013-08-02Grant Date: 2018-02-28
- Compounds to enhance cognitive functionPublication Number: ES-2666134-T3Priority Date: 2013-08-02Grant Date: 2018-05-03
- Compounds for enhancing the cognitive functionPublication Number: SI-3027606-T1Priority Date: 2013-08-02
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015014785&_cid=P11-MI5R7J-79014-1
Example 1 : Synthesis of (4R)-1 -[(5-chloro-1H-1,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 7.

1.1 Synthesis of tert-butyl 2-oxo-4-(3,4,5-trifluorophenyl)pyrrolidine-1 -carboxylate 3 and enantiomers.
To a solution of tert-butyl 2-oxo-2,5-dihydro-1 H-pyrrole-1-carboxylate 1 (10 g, 1 eq., 54.6 mmol) in dioxane/water (100 ml/30 ml) are added at room temperature (3,4,5-trifluorophenyl)boronic acid 2 (19.2 g, 2 eq., 109.2 mmol), cesium fluoride (24.9 g, 3 eq., 163.8 mmol), (±)-2,2′-bis(diphenyl-phosphino)-1 , 1′-binaphthyl (1.5 g, 4.5%, 2.5 mmol), potassium carbonate (22.6 g, 3 eq., 163.8 mmol) and chloro(1 ,5-cyclooctadiene)rhodium(l)dimer (0.82 g, 1.5%, 8.2 mmol). The mixture is heated at 1 10°C for 2 h. Solvent are removed under reduced pressure and the residue is purified by chromatography over silicagel (eluent: CI-^C^/MeOH/NI-^OH 96/3.5/0.5 v/v/v) to afford tert-butyl 2-oxo-4-(3,4,5-trifluorophenyl)pyrrolidine-1-carboxylate 3. The enantiomers are
resolved by chiral chromatography (chiralpak IC, 150*4.6 mm, eluent: heptane/AcOEt/diethylamine 80/20/0.1 v/v/v) to afford tert-butyl (4R)-2-oxo-4-(3,4,5-trifluorophenyl)pyrrolidine-1-carboxylate 3A (second eluted, 5.1 g), and its enantiomer tert-butyl (4S)-2-oxo-4-(3,4,5-trifluorophenyl)pyrrolidine-1-carboxylate 3B (first eluted, 5.2 g) as white solids.
Compound 3A:
Yield: 30%.
LC-MS (MH+): 316.
alphaD (MeOH, 25°C): -19.9.
1.2 Synthesis of (4R)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 4.
At 0°C, TFA (20 ml, 261 mmol) is added to a solution of tert-butyl (4R)-2-oxo-4-(3,4,5-trifluorophenyl)pyrrolidine-1-carboxylate 3A (8 g, 1 eq., 25.4 mmol) in dichloromethane (100 ml). The mixture is stirred at room temperature for 2 h. Then, TFA and solvent are removed under reduced pressure. The crude mixture is poured in an aqueous saturated solution of NaHCC>3 (100 ml) and extracted with AcOEt (3*200 ml). The combined organic extracts are dried over MgS04 and concentrated under reduced pressure. The conversion is total and the evaporation affords 5.5 g of (4R)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 4, which is used in the next step without any further purification.
LC-MS (MH+): 216; LC-MS (MKT): 214.
alphaD (MeOH, 22°C): -20.1.
1.3 Synthesis of (4R)-1 -(hydroxymethyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 5.
To a solution of (4R)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 4 (5.5 g, 1 eq., 25.6 mmol) in THF (20 ml) are added potassium tert-butoxide (0.049 g, 0.02 eq., 0.44 mmol) and paraformaldehyde (0.95 g, 1.2 eq., 31.1 mmol) at room temperature. After overnight stirring at 60°C, the mixture is quenched with brine (100 ml) and the aqueous phase is extracted with AcOEt (2*100 ml). The combined organic extracts are dried over MgS04 and concentrated under reduced pressure yielding 4.7 g of (4R)-1-(hydroxymethyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 5, which is used in the next step without any further purification.
LC-MS (MH+): 246.
H NMR (DMSO) δ 7.34 (dd, J-| =9.2 Hz, J2=6.8 Hz, 2 H), 5.87 (t, J=6.8 Hz, 1 H), 4.70 (m, 2 H), 3.78 (m, 1 H), 3.62 (m, 1 H), 3.40 (m, 1 H), 2.68 (m, 1 H), 2.43 (dd, J<l =16.6 Hz, J2=8.6 Hz, 1 H).
1.4 Synthesis of (4R)-1 -[(5-chloro-1 H-1 ,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluoro- phenyl)pyrrolidin-2-one 7.
1 ) To a cold solution (0°C) of (4R)-1-(hydroxymethyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 5 (4.7 g, 1 eq., 19.4 mmol) in CH2CI2 (200 mL) is added oxalyl chloride (3.7 ml, 2 eq., 38 mmol). After stirring for 30 minutes at 0°C, the reaction mixture is evaporated in vacuum yielding (4R)-1-(chloromethyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 6 which is dissolved in THF (100 ml) to afford Solution A.
2) To a cold solution (0°C) of 5-chloro-1 H-1 ,2,4-triazole (3.0 g, 1.5 eq., 29.1 mmol) in THF (100 ml) is added NaH 95% in mineral oil (0.9 g, 2 eq., 38.7 mmol). The reaction mixture is stirred during 30 minutes at 0°C to afford Solution B.
3) Solution A is added to solution B at 0°C and the reaction mixture is maintained under stirring overnight at room temperature. The mixture is quenched with water (100 ml) and extracted with AcOEt (2*100 mL). The combined organic extracts are washed with brine (100 ml), dried over MgS04 then concentrated under reduced pressure yielding 7 g of compound 7 as crude material. The crude residue is purified by chromatography on silicagel (eluent: CH2Cl2/MeOH/NH4OH 95/5/0.5 v/v/v) and recrystallized from iPr20/EtOH affording 1.6 g of (4R)-1-[(5-chloro-1 H-1 ,2,4-triazol-1-yl)methyl]-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one 7 as a white solid.
Yield: 25%.
LC-MS (MH+): 331/333.
H NMR (DMSO) δ 8.12 (s, 1 H), 7.32 (dd, J-| =9.2 Hz, J2=6.9 Hz, 2 H), 5.63 (d, J=1.5 Hz, 2 H), 3.81 (t, J=8.6 Hz, 1 H), 3.62 (t, J=8.4 Hz, 1 H), 3.39 (m, 1 H), 2.71 (dd, J<l =16.7 Hz, J2=8.8 Hz, 1 H), 2.54 (d, J=9.1 Hz, 1 H).
alphaD (MeOH, 25°C): + 9.2.



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | ABBV-552; ABBV552; SDI-118; SDI118 |
| Routes of administration | Oral[1] |
| Drug class | Synaptic vesicle glycoprotein 2A (SV2A) ligand[2] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1651179-19-9 |
| PubChem CID | 90467376 |
| ChemSpider | 129532952 |
| UNII | W3LYF2KQ6F |
| KEGG | D13077 |
| ChEMBL | ChEMBL5314929 |
| Chemical and physical data | |
| Formula | C13H10ClF3N4O |
| Molar mass | 330.70 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- “ABBV 552”. AdisInsight. 28 October 2024. Retrieved 26 February 2025.
- Botermans W, Koole M, Van Laere K, Savidge JR, Kemp JA, Sunaert S, et al. (2022). “SDI-118, a novel procognitive SV2A modulator: First-in-human randomized controlled trial including PET/fMRI assessment of target engagement”. Frontiers in Pharmacology. 13 1066447. doi:10.3389/fphar.2022.1066447. PMC 9887116. PMID 36733374.
- “Delving into the Latest Updates on Plosaracetam with Synapse”. Synapse. 22 February 2025. Retrieved 26 February 2025.
- “ABBV-552”. ALZFORUM. 28 February 2023. Retrieved 26 February 2025.
/////////Plosaracetam, ABBV-552, ABBV552, SDI-118, SDI118, ABBV 552, ABBV552, SDI 118, SDI118, W3LYF2KQ6F














