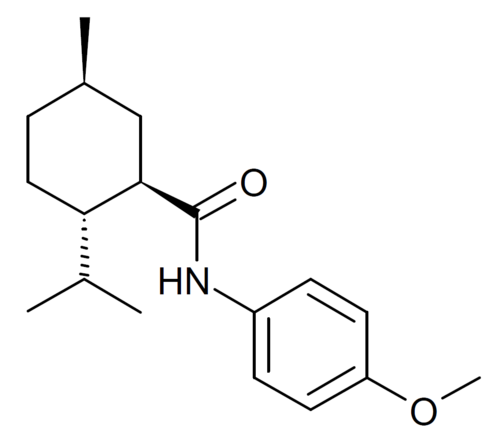
Acoltremon
CAS 68489-09-8
WeightAverage: 289.419
Monoisotopic: 289.204179113
Chemical FormulaC18H27NO2
FDA 2025, 5/28/2025, To treat the signs and symptoms of dry eye disease
Tryptyr |
WS 12
(1R,2S,5R)-N-(4-methoxyphenyl)-5-methyl-2-(propan-2-yl)cyclohexane-1-carboxamide
Fema No. 4681
N-(4-methoxyphenyl)-p-menthanecarboxamide
- OriginatorInstituto de Neurociencias de Alicante
- DeveloperAlcon; AVX Pharma
- ClassCyclohexanes; Ethers; Eye disorder therapies; Small molecules
- Mechanism of ActionTRPM8 protein stimulants
- RegisteredDry eyes
- 30 May 2025Alcon plans to launch Acoltremon for Dry eyes in USA in the third quarter of 2025
- 28 May 2025Registered for Dry eyes in USA (Ophthalmic) – First global approval
- 05 May 2025FDA assigns PDUFA action date of 30/05/2025 for Acoltremon for Dry eyes
Acoltremon sold under the brand name Tryptyr, is a medication used for the treatment of dry eye syndrome.[1]
PATENT
US 217370
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023114986&_fid=RU437402572
https://patentscope.wipo.int/search/en/detail.jsf?docId=US193167995&_cid=P11-MCE7BB-27500-1
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012032209&_fid=US193167995
Medical uses
Acoltremon was approved for medical use in the United States in May 2025, for the treatment of signs and symptoms associated with dry eye disease.[2]
Pharmacology
Acoltremon acts as a potent and selective activator (opener) of the TRPM8 calcium channel, which is responsible for the sensation of coldness produced by menthol.[3] It is slightly less potent as a TRPM8 activator compared to icilin, but is a much more selective TRPM8 ligand when compared to menthol.[4]
Society and culture
Legal status
Acoltremon was approved for medical use in the United States in May 2025.[5]
References
- ^ Jump up to:a b https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/217370s000lbl.pdf
- ^ “Novel Drug Approvals for 2025”. U.S. Food and Drug Administration (FDA). 29 May 2025. Archived from the original on 3 March 2025. Retrieved 29 May 2025.
- ^ Ma S, Gisselmann G, Vogt-Eisele AK, Doerner JF, Hatt H (October 2008). “Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels”. Pakistan Journal of Pharmaceutical Sciences. 21 (4): 370–378. PMID 18930858.
- ^ Kühn FJ, Kühn C, Lückhoff A (February 2009). “Inhibition of TRPM8 by icilin distinct from desensitization induced by menthol and menthol derivatives”. The Journal of Biological Chemistry. 284 (7): 4102–4111. doi:10.1074/jbc.M806651200. PMID 19095656.
- ^ “Alcon Announces FDA Approval of Tryptyr (acoltremon ophthalmic solution) 0.003% for the Treatment of the Signs and Symptoms of Dry Eye Disease” (Press release). Alcon. 28 May 2025. Archived from the original on 29 May 2025. Retrieved 29 May 2025 – via Business Wire.
External links
- Clinical trial number NCT05285644 for “Study Evaluating the Safety and Efficacy of AR-15512 (COMET-2)” at ClinicalTrials.gov
- Clinical trial number NCT05360966 for “Study Evaluating the Safety and Efficacy of AR-15512 (COMET-3)” at ClinicalTrials.gov
| molecular structure | |
| 3D representation | |
| Clinical data | |
|---|---|
| Trade names | Tryptyr |
| Other names | AVX-012, WS-12 |
| License data | US DailyMed: Acoltremon |
| ATC code | None |
| Legal status | |
| Legal status | US: ℞-only[1] |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 68489-09-8 |
| PubChem CID | 11266244 |
| DrugBank | DB19202 |
| ChemSpider | 9441255 |
| UNII | 1L7BVT4Z4Z |
| KEGG | D13125 |
| ChEMBL | ChEMBL2441929 |
| CompTox Dashboard (EPA) | DTXSID10460636 |
| Chemical and physical data | |
| Formula | C18H27NO2 |
| Molar mass | 289.419 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
- [1]. Beck B, et al. Prospects for prostate cancer imaging and therapy using high-affinity TRPM8 activators. Cell Calcium. 2007 Mar;41(3):285-94. [Content Brief][2]. Ma S, et al. Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak J Pharm Sci. 2008 Oct;21(4):370-8. [Content Brief]
///////Acoltremon, FDA 2025, APPROVALS 2025, WS-12, WS 12, Fema No. 4681, Tryptyr, 1L7BVT4Z4Z, AR-15512














