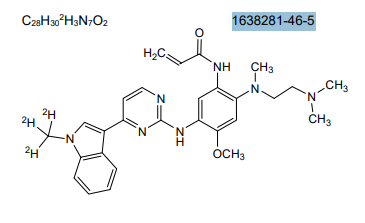
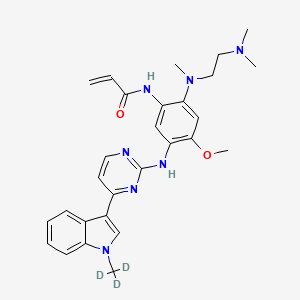
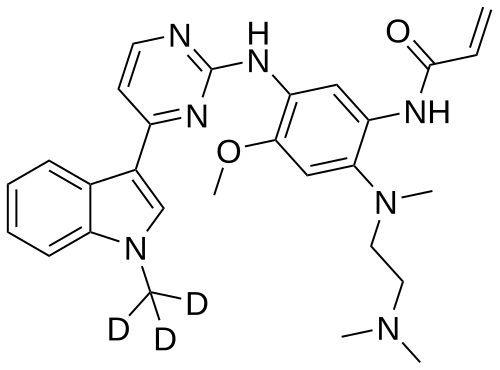
Asandeutertinib, Osimertinib-d3
CAS 1638281-46-5
- 9EKD2E8BM5
- N-(2-(2-(dimethylamino)ethyl-methylamino)-4-methoxy-5-((4-(1-(trideuteriomethyl)indol-3-yl)pyrimidin-2-yl)amino)phenyl)prop-2-enamide
- N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-[1-(trideuteriomethyl)indol-3-yl]pyrimidin-2-yl]amino]phenyl]prop-2-enamide
N-[2-{2-(dimethylamino)ethylamino}-4-methoxy-5-({4-[1-(2H3)methyl-1H-indol-3-yl]pyrimidin-2-
yl}amino)phenyl]prop-2-enamide
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, antineoplastic
MF C28H30. 2H3. N7O2, C28H30D3N7O2 MW 502.6 g/mol
Asandeutertinib is an investigational new drug that is being evaluated for the treatment of cancer. It is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with antineoplastic properties.[1][2] Developed by TYK Medicines, this small molecule drug is currently being investigated for the treatment of non-small cell lung cancer (NSCLC), particularly in patients with EGFR mutations.[1][3]
PAT
- 2-(2,4,5-substituted aniline) pyrimidine derivative, pharmaceutical composition and use thereofPublication Number: US-10414756-B2Priority Date: 2014-08-15Grant Date: 2019-09-17
- 2-(2,4,5-substituted aniline) pyrimidine derivative, pharmaceutical composition and use thereofPublication Number: US-2018016258-A1Priority Date: 2014-08-15
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US210080627&_cid=P21-MFT3HT-86141-1
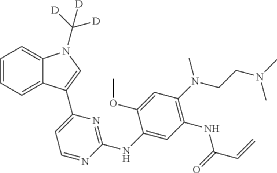
Embodiment 3A
N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-(D3-methyl)indol-3-yl)pyrimidin-2-yl]amino}phenyl)-2-acrylamide
| Under ice bath condition, to N 1-(2-dimethylaminoethyl)-5-methoxy-N 1-methyl-N 4-[4-(1-[D 3-methylindol]-3-yl)pyrimidin-2-yl]phenyl-1,2,4-triamine (intermediate 3, 20 g) in THF (200 mL) and water (20 mL), was added 6.9 g NaOH. Acryloyl chloride 4.05 g was added while stirring, the reaction mixture was stirred for 30 min at room temperature, then stirred for 1 h at room temperature. After the result of TLC showed that the reaction was complete, 200 mL water and 20 mL aqueous ammonia were added into the reaction mixture, the solid was precipitated and filtered out. The solid was collected and washed with water, dried for 8 h at 50° C. to deliver the title compound (yield 87%). |



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- “Asandeutertinib”. PatSnap.
- “Asandeutertinib”. IUPHAR/BPS Guide to PHARMACOLOGY.
- Han B, Zhang W, Wu L, Chen B, Zhao Y, Liu J, et al. (October 2024). “P1. 12A. 07 A Phase 1 Study of TY-9591 in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients with EGFR Positive Mutation”. Journal of Thoracic Oncology. 19 (10): S195. doi:10.1016/j.jtho.2024.09.353.
| Clinical data | |
|---|---|
| Other names | Runnor-9591, TY 9591 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1638281-46-5 |
| PubChem CID | 87056175 |
| IUPHAR/BPS | 13201 |
| ChemSpider | 129431787 |
| UNII | 9EKD2E8BM5 |
| Chemical and physical data | |
| Formula | C28H30D3N7O2 |
| Molar mass | 502.636 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
////////////Asandeutertinib, antineoplastic, 9EKD2E8BM5, Osimertinib-d3














