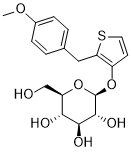
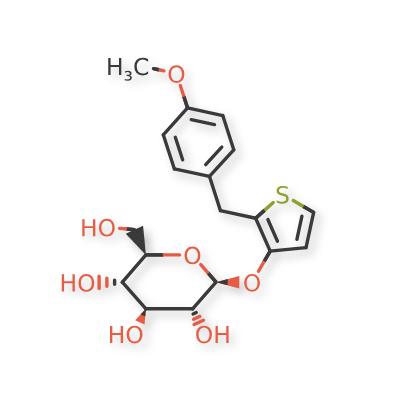
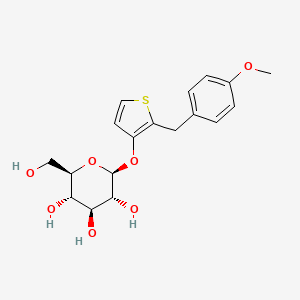
Atigliflozin
CAS 647834-15-9
Chemical Formula: C18H22O7S
Exact Mass: 382.1086
Molecular Weight: 382.43
AVE 2268; AVE-2268; AVE2268; Y0H7UPE4WJ
(2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-((2-(4-methoxybenzyl)thiophen-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triol
Atigliflozin (AVE-2268) is an orally active and selective SGLT-2 inhibitor, with IC50s of 10 nM and 8.2 μM for hSGLT-2 and hSGLT-1) respectively. Atigliflozin can lower the blood glucose and improve the impaired oral glucose tolerance. Atigliflozin can be used for research of type II diabetes mellitus.
Patent
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: KR-20240160678-APriority Date: 2008-08-06
- Use of thiophene glycoside derivatives for producing medicaments for treatment of hypertensionPublication Number: WO-2009138195-A2Priority Date: 2008-05-16
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: KR-20200118243-APriority Date: 2008-08-06
- Treatment of diabetes in patients who are inadequate for metformin treatmentPublication Number: JP-2021035998-APriority Date: 2008-08-06
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: US-2021093633-A1Priority Date: 2008-08-06
- Treatment of diabetes in patients unsuitable for metformin treatmentPublication Number: JP-2023011007-APriority Date: 2008-08-06
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: US-2022323434-A1Priority Date: 2008-08-06
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: US-2018271859-A1Priority Date: 2008-08-06
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: US-2019105321-A1Priority Date: 2008-08-06
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: US-8853156-B2Priority Date: 2008-08-06Grant Date: 2014-10-07
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: US-9486526-B2Priority Date: 2008-08-06Grant Date: 2016-11-08
- Treatment for diabetes in patients inappropriate for metformin therapyPublication Number: WO-2010015664-A1Priority Date: 2008-08-06
SYN
https://www.sciencedirect.com/science/article/abs/pii/S022352342400223X
Atigliflozin is developed by Sanofi and is currently in phase II clinical development. It is used for the treatment of T2DM (IC50= 13 nmol/L)[74]. In mice, Atigliflozin led to a rise in urinary glucose excretion that was dependent on the dosage administered (ID3030=79±8.1 mg/kg p.o.). Similarly, in rats, Atigliflozin caused a dose-dependent increase in UGE(ID= 39.8±4.0 mg/kg p.o.). When glucose was administered intraperitoneally, Atigliflozin was found to be more effective in reducing blood glucose levels in mice (IDorally administered glucose (ID5050= 13.2±3.9 mg/kg) compared to =26.1±3.9 mg/kg). This suggests that Atigliflozin does not have an impact on SGLT 1 in the gut in vivo, which
aligns with its very low affinity to SGLT1 in vitro Additionally, studies have demonstrated that the combined use of metformin and Atigliflozin can effectively lower glucose levels by inhibiting the body’s natural glucose production. This coapplication may offer a sustainable solution for improving glycemic control in in dividuals with T2DM [75].
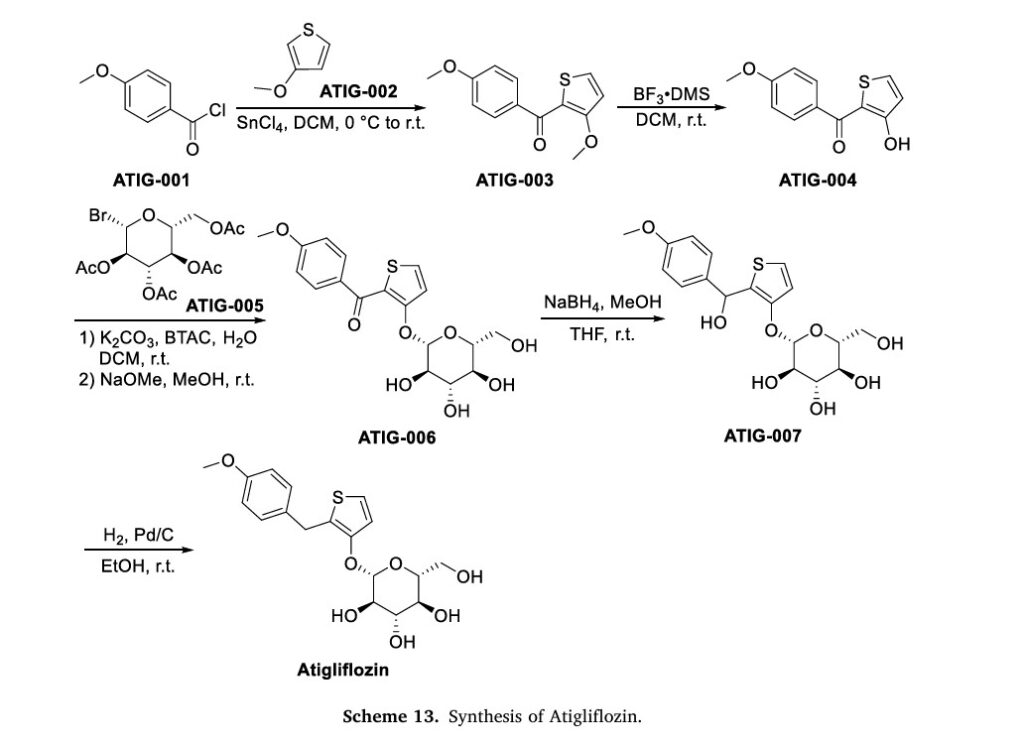
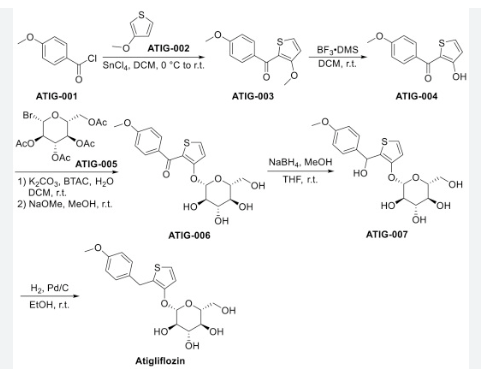
The original synthesis route of Atigliflozin is showed in Scheme 13 [76,77]. Friedel-Crafts acylation of 4-methoxybenzoyl chloride (ATIG-001) with 3-methoxythiophene (ATIG-002) catalyzed by SnCl114to give the ketone ATIG-003. In the presence of borane-methyl sulfide (DMS) complex, ATIG-003 is demethylated to give the thiophenol ATIG-004. Next, nucleophilic substitution of ATIG-004 with 2,3,4,
6-tetra-O-acetyl αD-glucopyranosyl bromide (ATIG-005), followed by hydrolysis in the presence of sodium methanolate give ether ATIG-006. ATIG-006 is reduced by sodium borohydride to give the alcohol ATIG-007. Finally, further reduction of ATIG-007 catalyzed by Pd/C with H2 provides Atigliflozin.
[74] M. Bickel, H. Brummerhop, W. Frick, H. Glombik, A.W. Herling, H.O. Heuer,
O. Plettenburg, S. Theis, U. Werner, W. Kramer, Effects of AVE2268, a substituted
glycopyranoside, on urinary glucose excretion and blood glucose in mice and rats,
Arzneimittelforschung 58 (2008) 574–580.
[75] S. Neschen, M. Scheerer, A. Seelig, P. Huypens, J. Schultheiss, M. Wu, W. Wurst,
B. Rathkolb, K. Suhre, E. Wolf, J. Beckers, M. Hrab´e de Angelis, Metformin
supports the antidiabetic effect of a sodium glucose cotransporter 2 inhibitor by
suppressing endogenous glucose production in diabetic mice, Diabetes 64 (2015)
284–290.
[76] G. Heiner, F. Wendelin, H. Hubert, K. Werner, Novel Thiophenylglycoside
Derivatives, Methods for Production Thereof, Medicaments Comprising Said
Compounds and Use Thereof, 2014 WO2004007517A1.
[77] H. Glombik, W. Frick, H. Heuer, W. Kramer, Thiophene Glycoside Derivatives,
Processes for the Preparation, Medicaments Comprising These Compounds, and the
Use Thereof, 2010 US7666848B2.
- The magic of small structure differences in a sodium‐glucose cotransporter drug discovery project—14C‐labelled drug candidates in a key‐differentiating studyPublication Name: Journal of Labelled Compounds and RadiopharmaceuticalsPublication Date: 2020-07-14PMID: 32633850DOI: 10.1002/jlcr.3869
- Metformin Supports the Antidiabetic Effect of a Sodium Glucose Cotransporter 2 Inhibitor by Suppressing Endogenous Glucose Production in Diabetic MicePublication Name: DiabetesPublication Date: 2014-07-28PMID: 25071027DOI: 10.2337/db14-0393
- Energy loss via urine and faeces – a combustive analysis in diabetic rats and the impact of antidiabetic treatment on body weightPublication Name: Diabetes, Obesity and MetabolismPublication Date: 2012-11-22PMID: 23121319DOI: 10.1111/dom.12030
- Effects of AVE2268, a Substituted Glycopyranoside, on Urinary Glucose Excretion and Blood Glucose in Mice and RatsPublication Name: Arzneimittel-ForschungPublication Date: 2011-12-19PMID: 19137908DOI: 10.1055/s-0031-1296559
- [1]. Schudok M, et al. The magic of small structure differences in a sodium-glucose cotransporter drug discovery project-14 C-labelled drug candidates in a key-differentiating study. J Labelled Comp Radiopharm. 2021 Feb;64(2):73-76. [Content Brief][2]. Bickel M, et al. Effects of AVE2268, a substituted glycopyranoside, on urinary glucose excretion and blood glucose in mice and rats. Arzneimittelforschung. 2008;58(11):574-80. [Content Brief]
////////// Atigliflozin, AVE 2268, AVE-2268, AVE2268, Y0H7UPE4WJ














