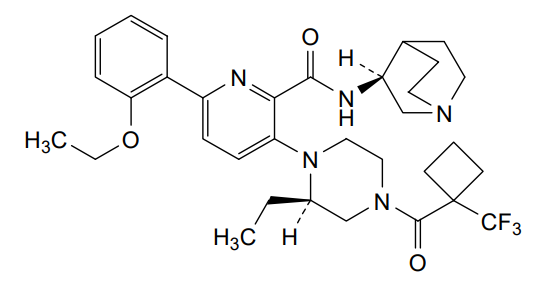
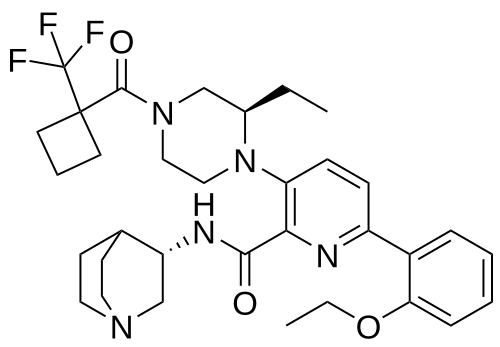
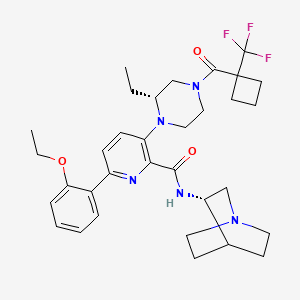
Atumelnant
CAS 2392970-97-5
MF C33H42F3N5O3 MW 613.7 g/mol
CRN04894, NR57FH6U1N
CRINETICS PHARMA, Orphan Drug Status, Congenital adrenal hyperplasia
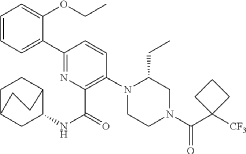
N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-6-(2-ethoxyphenyl)-3-[(2R)-2-ethyl-4-[1-(trifluoromethyl)cyclobutanecarbonyl]piperazin-1-yl]pyridine-2-carboxamide
N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-6-(2-ethoxyphenyl)-3-{(2R)-2-ethyl-4-[1-(trifluoromethyl) cyclobutane-1-carbonyl]piperazin-1-yl}pyridine-2-carboxamide
Adrenocorticotropic hormone receptor antagonist
- OriginatorCrinetics Pharmaceuticals
- ClassAmides; Antineoplastics; Antisecretories; Benzene derivatives; Cyclobutanes; Ethers; Fluorocarbons; Ketones; Piperazines; Pyridines; Quinuclidines; Small molecules
- Mechanism of ActionMelanocortin type 2 receptor antagonists
- Orphan Drug StatusYes – Congenital adrenal hyperplasia
- Phase IICongenital adrenal hyperplasia; Cushing syndrome
- No development reportedEctopic ACTH syndrome
- 21 Aug 2025Atumelnant receives Orphan Drug status for Congenital adrenal hyperplasia in the US
- 07 Aug 2025Crinetics pharmaceuticals plans phase II/III clinical trial for Cushing’s disease in 1H 2026
- 08 May 2025Crinetics Pharmaceuticals plans the phase III CALM-CAH trial for Congenital adrenal hyperplasia (In adults) (PO), in the second half of 2025
Atumelnant (INNTooltip International Nonproprietary Name; developmental code name CRN04894) is an investigational new drug developed by Crinetics Pharmaceuticals for the treatment of adrenocorticotropic hormone (ACTH)-dependent endocrine disorders.[1] It is a selective antagonist of the melanocortin type 2 receptor (MC2R), also known as the ACTH receptor, which is primarily expressed in the adrenal glands.[1][2] The drug is orally active.[1] Atumelnant is being evaluated to treat conditions such as congenital adrenal hyperplasia (CAH) and ACTH-dependent Cushing’s syndrome caused for example by pituitary adenomas.[3]
Atumelnant is an orally bioavailable nonpeptide antagonist of the adrenocorticotropic hormone (ACTH) receptor (ACTHR; melanocortin receptor 2; MC2R), with potential steroid hormone production inhibitory activity. Upon oral administration, atumelnant competes with ACTH for receptor binding to MC2R in the adrenal cortex and inhibits ACTH signaling. This may inhibit the synthesis and secretion of steroid hormones. MC2R, a member of the melanocortin receptor subfamily of type 1 G protein-coupled receptors, plays a key role in adrenal steroidogenesis.
PAPER
Discovery of CRN04894: A Novel Potent Selective MC2R Antagonist
Publication Name: ACS Medicinal Chemistry Letters
Publication Date: 2024-03-19, PMCID: PMC11017392, PMID: 38628803
DOI: 10.1021/acsmedchemlett.3c00514
PATENTS
- Melanocortin subtype-2 receptor antagonists and uses thereofPublication Number: IL-279152-B2Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: US-2024300920-A1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor antagonists and uses thereofPublication Number: IL-279152-B1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: JP-2024009837-APriority Date: 2018-06-05
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: KR-102695210-B1Priority Date: 2018-06-05Grant Date: 2024-08-13
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: US-2024109866-A1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: CN-112533904-BPriority Date: 2018-06-05Grant Date: 2024-10-29
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: US-10981894-B2Priority Date: 2018-06-05Grant Date: 2021-04-20
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: US-2021002254-A1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: US-2021238164-A1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: US-11566015-B2Priority Date: 2018-06-05Grant Date: 2023-01-31
- Melanocortin subtype-2 receptor (MC2R) antagonists and their usesPublication Number: JP-7359783-B2Priority Date: 2018-06-05Grant Date: 2023-10-11
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: US-2020216415-A1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: US-10766877-B2Priority Date: 2018-06-05Grant Date: 2020-09-08
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: CN-112533904-APriority Date: 2018-06-05
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: EP-3802500-A1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: KR-20210005995-APriority Date: 2018-06-05
- Melanocortin subtype-2 receptor (MC2R) antagonists for the treatment of diseasePublication Number: CN-117043146-APriority Date: 2021-03-19
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: US-10562884-B2Priority Date: 2018-06-05Grant Date: 2020-02-18
- Melanocortin subtype-2 receptor (MC2R) antagonists and uses thereofPublication Number: US-10604507-B2Priority Date: 2018-06-05Grant Date: 2020-03-31
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: US-2019367481-A1Priority Date: 2018-06-05
- Melanocortin subtype-2 receptor (mc2r) antagonists and uses thereofPublication Number: US-2020010452-A1Priority Date: 2018-06-05
Melanocortin subtype-2 receptor (mc2r) antagonist for the treatment of diseasePublication Number: US-2022313691-A1Priority Date: 2021-03-19 - Melanocortin subtype-2 receptor (mc2r) antagonist for the treatment of diseasePublication Number: WO-2022197798-A1Priority Date: 2021-03-19
- Melanocortin subtype-2 receptor (mc2r) antagonist for the treatment of diseasePublication Number: TW-202302108-APriority Date: 2021-03-19
- Melanocortin subtype-2 receptor (mc2r) antagonist for the treatment of diseasePublication Number: AU-2022240609-A1Priority Date: 2021-03-19
- Melanocortin subtype-2 receptor (mc2r) antagonist for the treatment of diseasePublication Number: EP-4308553-A1Priority Date: 2021-03-19
- Melanocortin subtype-2 receptor (mc2r) antagonist for the treatment of acth-dependent cushing’s syndromePublication Number: WO-2024211343-A1Priority Date: 2023-04-05
- Crystalline melanocortin subtype-2 receptor (mc2r) antagonistPublication Number: TW-202430167-APriority Date: 2022-12-16
- Crystalline melanocortin subtype-2 receptor (mc2r) antagonistPublication Number: US-2024208963-A1Priority Date: 2022-12-16
- Crystalline melanocortin subtype-2 receptor (mc2r) antagonistPublication Number: WO-2024130091-A1Priority Date: 2022-12-16
- Treatment of congenital adrenal hyperplasia and polycystic ovary syndromePublication Number: WO-2023163945-A1Priority Date: 2022-02-2
SYN
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US278278493&_cid=P22-MFXDN2-76849-1
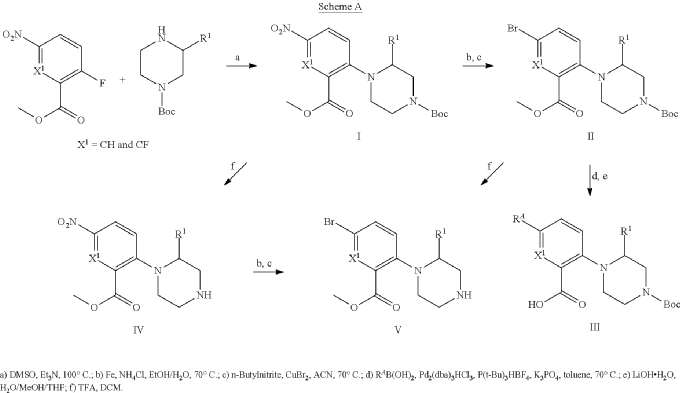
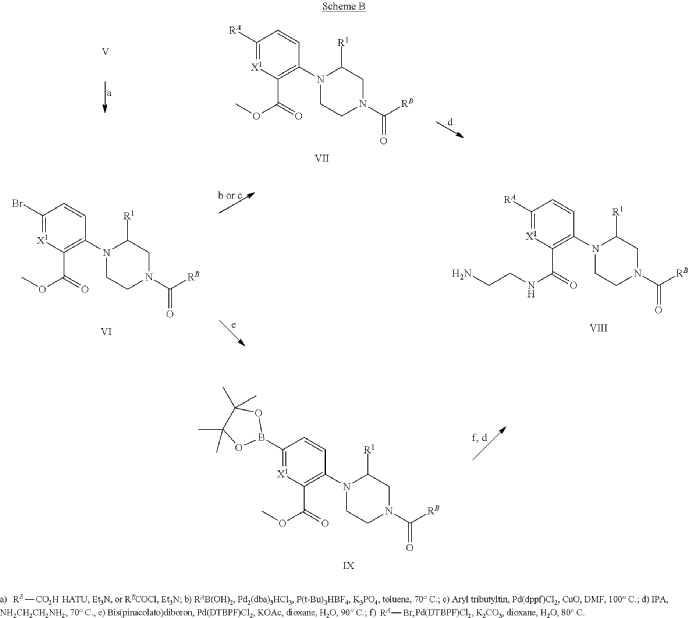
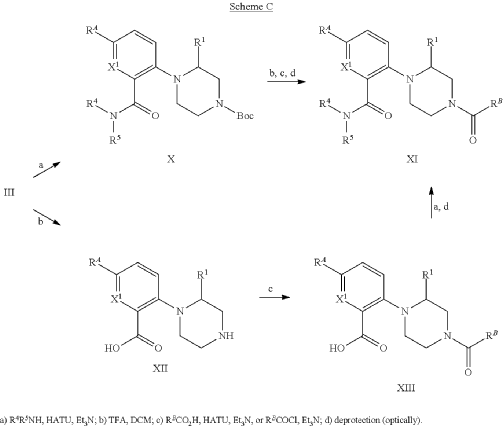
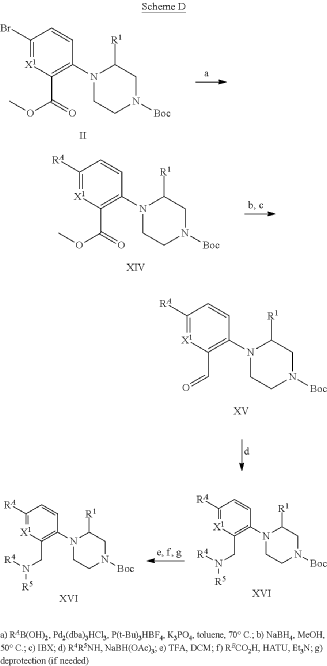
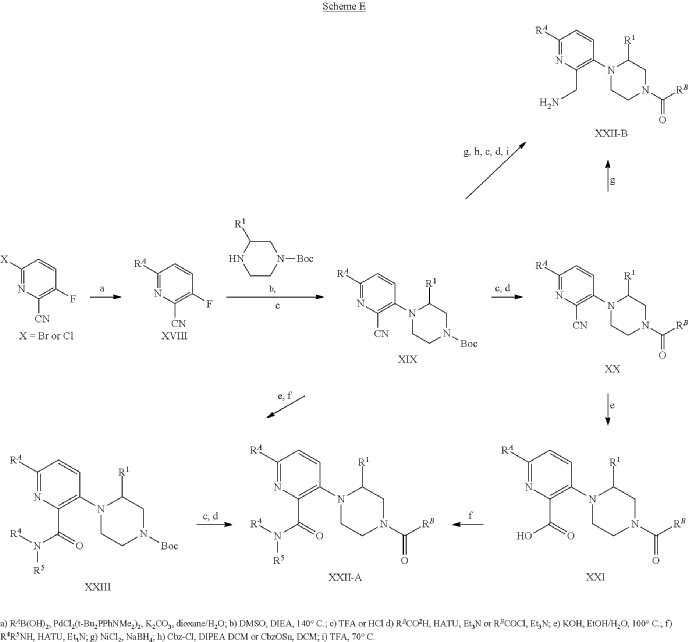
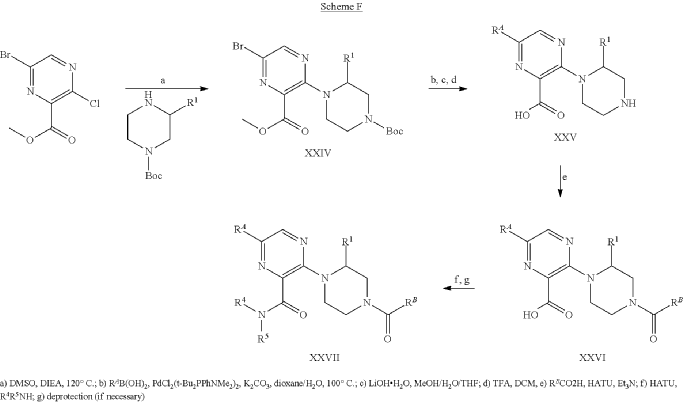
Example 31: N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-6-(2-ethoxyphenyl)-3-[(2R)-2-ethyl-4-[1-(trifluoromethyl)cyclobutanecarbonyl]piperazin-1-yl]pyridine-2-carboxamide (Compound 1-410)
Step 31-1, Preparation of 6-(2-ethoxyphenyl)-3-[(2R)-2-ethyl-4-[1-(trifluoromethyl)cyclobutanecarbonyl]piperazin-1-yl]pyridine-2-carboxylic acid
Step 31-2, Preparation of N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-6-(2-ethoxyphenyl)-3-[(2R)-2-ethyl-4-[1-(trifluoromethyl)cyclobutanecarbonyl]piperazin-1-yl]pyridine-2-carboxamide



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- “Crinetics Pharmaceuticals”. AdisInsight. 21 January 2025. Retrieved 25 February 2025.
- “Atumelnant (CRN04894)”. crinetics.com. 14 August 2020.
- Varlamov EV, Gheorghiu ML, Fleseriu M (December 2024). “Pharmacological management of pituitary adenomas – what is new on the horizon?”. Expert Opinion on Pharmacotherapy. 26 (2): 119–125. doi:10.1080/14656566.2024.2446625. PMID 39718553.
| Clinical data | |
|---|---|
| Other names | CRN04894 |
| Routes of administration | Oral[1] |
| Drug class | Melanocortin MC2 receptor antagonist[1] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2392970-97-5 |
| PubChem CID | 146361282 |
| IUPHAR/BPS | 13339 |
| ChemSpider | 129750231 |
| UNII | NR57FH6U1N |
| KEGG | D13102 |
| Chemical and physical data | |
| Formula | C33H42F3N5O3 |
| Molar mass | 613.726 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
////////Atumelnant, CRN04894, CRN 04894, NR57FH6U1N, CRINETICS PHARMA, Orphan Drug Status, Congenital adrenal hyperplasia, PHASE 3














