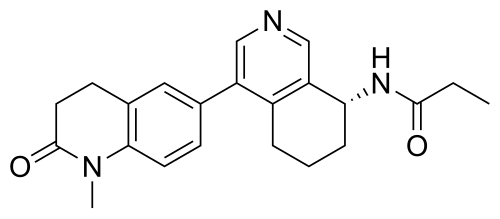
Baxdrostat
- NF3P9Z8J5Y
- CIN-107
- RO6836191
- 363.5 g/mol
WeightAverage: 363.461
Monoisotopic: 363.194677057
Chemical FormulaC22H25N3O2
N-[(8R)-4-(1-methyl-2-oxo-3,4-dihydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl]propanamide
- (+)-(R)-N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide
- N-((8R)-5,6,7,8-Tetrahydro-4-(1,2,3,4-tetrahydro-1-methyl-2-oxo-6-quinolinyl)-8-isoquinolinyl)propanamide
- N-[(8R)-4-(1-methyl-2-oxo-3,4-dihydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl]propanamide
- Propanamide, N-((8R)-5,6,7,8-tetrahydro-4-(1,2,3,4-tetrahydro-1-methyl-2-oxo-6-quinolinyl)-8-isoquinolinyl)-
Baxdrostat is an investigational drug that is being evaluated for the treatment of hypertension.[1] It is an aldosterone synthase inhibitor.[2][3]
Baxdrostat is under investigation in clinical trial NCT06344104 (A Phase III Study to Investigate the Efficacy and Safety of Baxdrostat in Asian Participants With Uncontrolled Hypertension on Two or More Medications Including Participants With Resistant Hypertension).
LIT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US76841362&_cid=P21-MEZ3MG-55484-1
Example 3-1
(+)-(R)—N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide
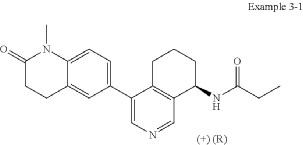
In analogy to the procedures described for the preparation of intermediate A-2 [E] and for the preparation of intermediate B-1, Suzuki reaction of (+)-(R)-4-bromo-5,6,7,8-tetrahydroisoquinolin-8-amine (intermediate B-3b) with 1-methyl-6-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-3,4-dihydro-1H-quinolin-2-one (intermediate A-1) gave (R)-6-(8-amino-5,6,7,8-tetrahydroisoquinolin-4-yl)-1-methyl-3,4-dihydroquinolin-2(1H)-one and after subsequent reaction with propionyl chloride the title compound as colorless solid. MS: 364.2 (M+H +).
Pat
CN 117247371
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN418385740&_cid=P12-MEZHY3-66430-1
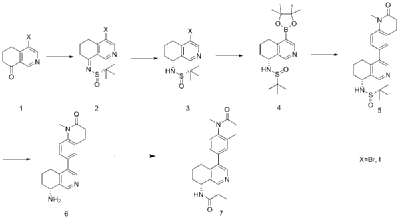
| Example 1 |
| Step A |
| Dissolve 4-bromo-6,7-dihydroisoquinolin-8(5H)-one (1.56 g, 6.9 mmol) and (S)-tert-butylsulfenamide (2.51 g, 20.7 mmol) in 20 mL of tetrahydrofuran. Add ethyl titanate (10.08 mL, 48.28 mmol). Heat to 65°C and stir for 48 hours. Cool to room temperature, add ethyl acetate and water, stir for 15 minutes, and remove the resulting solid by filtration. Separate the liquids, dry the organic phase over anhydrous sodium sulfate, filter, and evaporate to dryness under reduced pressure to obtain the crude product (S,Z)-N-(4-bromo-6,7-dihydroisoquinolin-8(5H)-tert-butylsulfenimide), which is used directly in the next step. |
| Step B |
| Compound (S,Z)-N-(4-bromo-6,7-dihydroisoquinoline-8(5H)-tert-butylsulfonyl imide) (1.98 g, 6 mmol) was dissolved in 15 mL of tetrahydrofuran and cooled to -45°C. Sodium borohydride (0.34 g, 9.0 mmol) was added, and the mixture was allowed to return to room temperature and stirred for 18 hours. The mixture was quenched with ice water and extracted with dichloromethane. The resulting organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The residue was purified by column chromatography to obtain compound (S)-N-(4-bromo-6,7-dihydroisoquinoline-8(5H))-tert-butylsulfonyl imide (755 mg, 38% yield). LC/MS (ESI): m/z = 331.2 [M+H] + . |
| Step C |
| To a mixture of (S)-N-(4-bromo-6,7-dihydroisoquinoline-8(5H))-tert-butylsulfonimide (0.66 g, 2 mmol), pinacol diboronate (1.05 g, 2.1 mmol), and AcOK (0.578 g, 6 mmol) in toluene (10 mL) was added Pd(dppf)Cl 2 (0.144 g, 0.2 mmol). The mixture was degassed and stirred at 130 ° C for 3 hours. The reaction mixture was filtered and concentrated to give a residue. EtOAc (15 mL) and water (10 mL) were added to the residue. The organic phase was washed with brine (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated to give a residue. The residue was purified by column chromatography (SiO 2 ) and eluted with 30-40% ethyl acetate in petroleum ether to afford (S)-N-tert-butylsulfonamido-6,7-dihydroisoquinolin-8(5H)-4-boronic acid pinacol ester (0.45 g, 60% yield). LC/MS (ESI): m/z = 378.3 [M+H] + . |
| Step D |
| To a reaction flask, add 6-bromo-1-methyl-3,4-dihydroquinolin-2(1H)-one (0.29 g, 1.2 mmol), (S)-N-tert-butylsulfonamido-6,7-dihydroisoquinolin-8(5H)-4-boronic acid pinacol ester (0.42 g, 1.26 mmol), bistriphenylphosphine palladium dichloride (84 mg, 0.12 mmol), cuprous iodide (38 mg, 0.2 mmol), triethylamine (1.01 g, 10.0 mmol), and 15 mL of N,N-dimethylformamide. The atmosphere was purged with nitrogen three times and the reaction was stirred at 90°C overnight. After cooling to room temperature, the reaction mixture was diluted with ethyl acetate and water, and extracted with ethyl acetate. The resulting organic phase was washed with water and saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The residue was purified by column chromatography to afford (S)-2-methyl-N-((R)-4-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)tert-butylsulfonimide (0.37 g, 74% yield) as a yellow solid. LC/MS (ESI): m/z = 411.5 [M+H] + . |
| Step E |
| Compound (S)-2-methyl-N-((R)-4-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)tert-butylsulfonimide (0.33 g, 0.80 mmol) was dissolved in 1 mL of dichloromethane, and 1 mL of trifluoroacetic acid was added. The mixture was stirred and reacted for 1 hour. The reaction solution was concentrated under reduced pressure. The residue was purified by reverse preparative column chromatography to obtain compound (R)-6-(8-amino-5,6,7,8-tetrahydroisoquinolin-4-yl)-1-methyl-3,4-dihydroquinolin-2(1H)-one (0.24 g, 97% yield). LC/MS (ESI): m/z = 307.1 [M+H] + . |
| Step F |
| To a reaction flask, add (R)-6-(8-amino-5,6,7,8-tetrahydroisoquinolin-4-yl)-1-methyl-3,4-dihydroquinolin-2(1H)-one (100 mg, 0.33 mmol), triethylamine (51 mg, 0.5 mmol), and 4 ml of tetrahydrofuran. After cooling in an ice-water bath, slowly add a solution of propionyl chloride (46.25 mg, 0.5 mmol) in 0.5 ml of tetrahydrofuran dropwise. Stirring is continued for 4 hours after addition. The reaction mixture is quenched with methanol and evaporated to dryness under reduced pressure. The residue is purified by column chromatography to obtain the target compound, Baxdrostat (46 mg, 38% yield). LC/MS(ESI):m/z=363.1[M+H]+.H NMR(400MHz, CDCl3)ppm 1.22(t,3H)1.79(s,3H)2.07(s,1H)2.28(q,2H)2.43-2.68(m,2H)2.71(t,2H)2.82-3.12(m,2H) 3.40(s,3H)5.34(d,1H)5.78(d,1H)7.05(d,1H)7.09(s,1H)7.17(d,1H)8.28(s,1H)8.49(s,1H) |
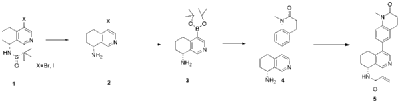
| Example 2 |

| Step A |
| Compound (S)-N-(4-bromo-6,7-dihydroisoquinolin-8(5H))-tert-butylsulfonylimide (1.65 g, 5 mmol) was dissolved in 20 mL of dichloromethane, and 20 mL of trifluoroacetic acid was added. The mixture was stirred and reacted for 1 hour. The reaction solution was concentrated under reduced pressure. The residue was purified by reverse-phase preparative column chromatography to obtain compound (R)-4-bromo-5,6,7,8-tetrahydroisoquinolin-8-amine (1.07 g, 94% yield). LC/MS (ESI): m/z = 226.0 [M+H] + . |
| Step B |
| To a mixture of (R)-4-bromo-5,6,7,8-tetrahydroisoquinolin-8-amine (0.86 g, 3.8 mmol), pinacol diboron (2 g, 4 mmol), AcOK (1.10 g, 11.4 mmol) in toluene (10 mL) was added Pd(dppf)Cl 2 (0.27 g, 0.38 mmol). The mixture was degassed and stirred at 130 ° C for 3 hours. The reaction mixture was filtered and concentrated to give a residue. EtOAc (10 mL) and water (10 mL) were added to the residue. The organic phase was washed with brine (10 mL), dried over anhydrous sodium sulfate, filtered and concentrated to give a residue. The residue was purified by column chromatography (SiO 2 ) and eluted with 30-40% ethyl acetate in petroleum ether to afford (R)-8-amino-5,6,7,8-tetrahydroisoquinoline-4-boronic acid pinacol ester (0.68 g, 65% yield). LC/MS (ESI): m/z = 274.1 [M+H] + . |
| Step C |
| To a reaction flask, add 6-bromo-1-methyl-3,4-dihydroquinolin-2(1H)-one (0.72 g, 3.0 mmol), (R)-8-amino-5,6,7,8-tetrahydroisoquinolin-4-boronic acid pinacol ester (0.99 g, 3.6 mmol), bistriphenylphosphine palladium dichloride (210 mg, 0.3 mmol), and potassium phosphate monohydrate (204 mg, 0.9 mmol). Dissolve the mixture in dioxane and water (9:1, 30 mL). Replace the atmosphere with nitrogen three times and allow the mixture to react overnight at 90°C with stirring. Cool to room temperature, dilute the reaction solution with ethyl acetate and water, and extract with ethyl acetate. The resulting organic phase is then washed with water and saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The residue was purified by column chromatography to obtain (R)-6-(8-amino-5,6,7,8-tetrahydroisoquinolin-4-yl)-1-methyl-3,4-dihydroquinolin-2(1H)-one (0.81 g, 88% yield). LC/MS (ESI): m/z = 307.1 [M+H] + . The target compound, Baxdrostat, was then prepared using a method similar to the last step in Example 1. |
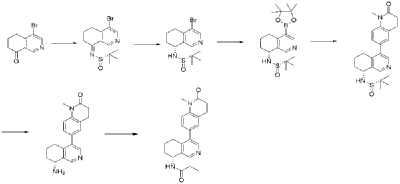
| Example 3 |
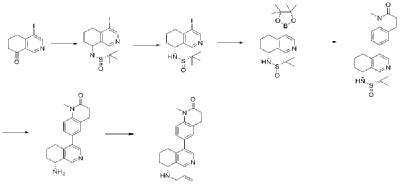
| Step A |
| 4-Bromo-6,7-dihydroisoquinolin-8(5H)-one (1.88 g, 6.9 mmol) and (S)-tert-butylsulfenamide (2.51 g, 20.7 mmol) were dissolved in 20 mL of tetrahydrofuran. Ethyl titanate (10.08 mL, 48.28 mmol) was added and the mixture was heated to 65°C with stirring for 48 hours. After cooling to room temperature, ethyl acetate and water were added and stirred for 15 minutes. The resulting solid was removed by filtration. The organic phase was separated and dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain the crude product (S,Z)-N-(4-bromo-6,7-dihydroisoquinolin-8(5H)-tert-butylsulfenimide), which was used directly in the next step. LC/MS (ESI): m/z = 376.2 [M+H] + . |
| Step B |
| Compound (S,Z)-N-(4-iodo-6,7-dihydroisoquinoline-8(5H)-tert-butylsulfonyl imide) (2.26 g, 6 mmol) was dissolved in 15 mL of tetrahydrofuran and cooled to -45°C. Sodium borohydride (0.36 g, 9.0 mmol) was added, and the mixture was allowed to return to room temperature and stirred for 18 hours. The mixture was quenched with ice water and extracted with dichloromethane. The resulting organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The residue was purified by column chromatography to obtain compound (S)-N-(4-iodo-6,7-dihydroisoquinoline-8(5H))-tert-butylsulfonyl imide (1.04 g, 46% yield). LC/MS (ESI): m/z = 378.0 [M+H] + . |
| Step C |
| To a mixture of (S)-N-(4-iodo-6,7-dihydroisoquinoline-8(5H))-tert-butylsulfonimide (0.76 g, 2 mmol), pinacol diboronate (1.05 g, 2.1 mmol), and AcOK (0.578 g, 6 mmol) in toluene (10 mL) was added Pd(dppf)Cl 2 (0.144 g, 0.2 mmol). The mixture was degassed and stirred at 130 ° C for 3 hours. The reaction mixture was filtered and concentrated to give a residue. EtOAc (15 mL) and water (10 mL) were added to the residue. The organic phase was washed with brine (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated to give a residue. The residue was purified by column chromatography (SiO 2 ) and eluted with 30-40% ethyl acetate in petroleum ether to afford (S)-N-tert-butylsulfonamido-6,7-dihydroisoquinolin-8(5H)-4-boronic acid pinacol ester (0.51 g, 68% yield). LC/MS (ESI): m/z = 378.2 [M+H] + . |
| The next three steps were carried out in the same manner as in Example 1 to prepare the target compound Baxdrostat. |
LIT
https://medicalxpress.com/news/2025-08-stubborn-high-blood-pressure-experimental.html
A new treatment has been shown to significantly lower blood pressure in people whose levels stay dangerously high, despite taking several existing medicines, according to the results of a Phase III clinical trial led by a UCL Professor. Globally, around 1.3 billion people have high blood pressure (hypertension), and in around half of cases the condition is uncontrolled or treatment resistant. These individuals face a much greater risk of heart attack, stroke, kidney disease, and early death. In the UK the number of people with hypertension is around 14 million.
The international BaxHTN trial, led by Professor Bryan Williams (UCL Institute of Cardiovascular Science), assessed the new drug baxdrostat—which is taken as a tablet—with participation from nearly 800 patients across 214 clinics worldwide.
Results were presented at the European Society of Cardiology (ESC) Congress 2025 in Madrid and were simultaneously published in the New England Journal of Medicine.
The trial results showed that, after 12 weeks, patients taking baxdrostat (1 mg or 2 mg once daily in pill form) saw their blood pressure fall by around 9-10 mmHg more than placebo—a reduction large enough to cut cardiovascular risk. About four in 10 patients reached healthy blood pressure levels, compared with fewer than two in 10 on placebo.
Principal Investigator, Professor Williams, who is presenting the results at ESC, said, “Achieving a nearly 10 mmHg reduction in systolic blood pressure with baxdrostat in the BaxHTN Phase III trial is exciting, as this level of reduction is linked to substantially lower risk of heart attack, stroke, heart failure and kidney disease.”
How baxdrostat works
Blood pressure is strongly influenced by a hormone called aldosterone, which helps the kidneys regulate salt and water balance.
Some people produce too much aldosterone, causing the body to hold onto salt and water. This aldosterone dysregulation pushes blood pressure up and makes it very difficult to control.
Addressing aldosterone dysregulation has been a key effort in research over many decades, but it has been so far difficult to achieve.
Baxdrostat works by blocking aldosterone production, directly addressing this driver of high blood pressure (hypertension).
Professor Williams, Chair of Medicine at UCL, said, “These findings are an important advance in treatment and in our understanding of the cause of difficult-to-control blood pressure.
“Around half of people treated for hypertension do not have it controlled, however this is a conservative estimate and the number is likely higher, especially as the target blood pressure we try to reach is now much lower than it was previously.
“In patients with uncontrolled or resistant hypertension, the addition of baxdrostat 1mg or 2mg once daily to background antihypertensive therapy led to clinically meaningful reductions in systolic blood pressure, which persisted for up to 32 weeks with no unanticipated safety findings.
“This suggests that aldosterone is playing an important role in causing difficult to control blood pressure in millions of patients and offers hope for more effective treatment in the future.”
Historically, higher-income Western countries were reported to have far higher levels of hypertension. However, largely due to changing diets (adding less salt to food), the numbers of people living with the condition is now far higher in Eastern and lower-income countries. More than half of those affected live in Asia, including 226 million people in China and 199 million in India.
Professor Williams added, “The results suggest that this drug could potentially help up to half a billion people globally—and as many as 10 million people in the UK alone, especially at the new target level for optimal blood pressure control.”



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1428652-17-8 |
| PubChem CID | 71535962 |
| IUPHAR/BPS | 12362 |
| ChemSpider | 76804781 |
| UNII | NF3P9Z8J5Y |
| KEGG | D12789 |
| ChEMBL | ChEMBL4113975 |
| Chemical and physical data | |
| Formula | C22H25N3O2 |
| Molar mass | 363.461 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
PATENTS
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: TW-201319054-APriority Date: 2011-09-23
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: TW-I576342-BPriority Date: 2011-09-23Grant Date: 2017-04-01
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: US-2013079365-A1Priority Date: 2011-09-23
- Bicyclic dihydroquinoline-2-one derivativesPublication Number: US-9353081-B2Priority Date: 2011-09-23Grant Date: 2016-05-31
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: WO-2013041591-A1Priority Date: 2011-09-23
- Novel bicyclic dihydroquinolin-2-one derivativesPublication Number: JP-2014526539-APriority Date: 2011-09-23
- Novel bicyclic dihydroquinolin-2-one derivativesPublication Number: JP-6012737-B2Priority Date: 2011-09-23Grant Date: 2016-10-25
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: KR-101723276-B1Priority Date: 2011-09-23Grant Date: 2017-04-04
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: KR-20140076591-APriority Date: 2011-09-23
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: NZ-620652-APriority Date: 2011-09-23
- New bicyclic dihydroquinolin-2-one derivativesPublication Number: CN-103827101-APriority Date: 2011-09-23
- Bicyclic dihydroquinolin-2-one derivativesPublication Number: CN-103827101-BPriority Date: 2011-09-23Grant Date: 2016-12-07
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: EP-2758388-A1Priority Date: 2011-09-23
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: EP-2758388-B1Priority Date: 2011-09-23Grant Date: 2018-02-21
- NEW BICYCLIC DERIVATIVES OF DIHYDROKINOLIN-2-ONPublication Number: HR-P20180592-T1Priority Date: 2011-09-23
- Methods of using aldosterone synthase inhibitorsPublication Number: US-2024277698-A1Priority Date: 2021-06-24
- How to use aldosterone synthase inhibitorsPublication Number: CN-117545482-APriority Date: 2021-06-24
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: AU-2012311582-A1Priority Date: 2011-09-23
- New bicyclic dihydroquinoline-2-one derivativesPublication Number: AU-2012311582-B2Priority Date: 2011-09-23Grant Date: 2017-07-06
- Bicyclic dihydroquinoline-2-one derivativesPublication Number: CA-2845170-CPriority Date: 2011-09-23Grant Date: 2019-08-13
References
- “Baxdrostat – CinCor Pharma”. AdisInsight. Springer Nature Switzerland AG.
- Dogra S, Shah S, Gitzel L, Pusukur B, Sood A, Vyas AV, Gupta R (July 2023). “Baxdrostat: A Novel Aldosterone Synthase Inhibitor for Treatment Resistant Hypertension”. Current Problems in Cardiology. 48 (11): 101918. doi:10.1016/j.cpcardiol.2023.101918. PMID 37399857. S2CID 259320969.
- Awosika A, Cho Y, Bose U, Omole AE, Adabanya U (October 2023). “Evaluating phase II results of Baxdrostat, an aldosterone synthase inhibitor for hypertension”. Expert Opinion on Investigational Drugs. 32 (11): 985–995. doi:10.1080/13543784.2023.2276755. PMID 37883217. S2CID 264517675.
- The selective aldosterone synthase inhibitor Baxdrostat significantly lowers blood pressure in patients with resistant hypertensionPublication Name: Frontiers in EndocrinologyPublication Date: 2022-12-09PMCID: PMC9780529PMID: 36568122DOI: 10.3389/fendo.2022.1097968
- Results from a phase 1, randomized, double-blind, multiple ascending dose study characterizing the pharmacokinetics and demonstrating the safety and selectivity of the aldosterone synthase inhibitor baxdrostat in healthy volunteersPublication Name: Hypertension research : official journal of the Japanese Society of HypertensionPublication Date: 2022-10-20PMCID: PMC9747611PMID: 36266539DOI: 10.1038/s41440-022-01070-4
- Preclinical and Early Clinical Profile of a Highly Selective and Potent Oral Inhibitor of Aldosterone Synthase (CYP11B2)Publication Name: Hypertension (Dallas, Tex. : 1979)Publication Date: 2017-01PMCID: PMC5142369PMID: 27872236DOI: 10.1161/hypertensionaha.116.07716
/////Baxdrostat, PHASE 3, NF3P9Z8J5Y, CIN 107, RO 6836191,














