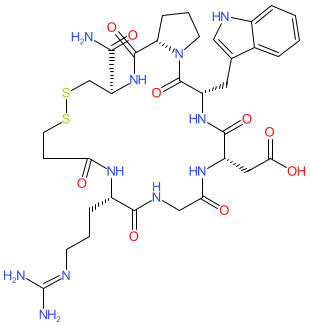
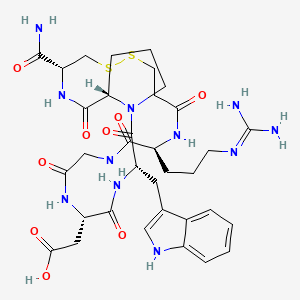
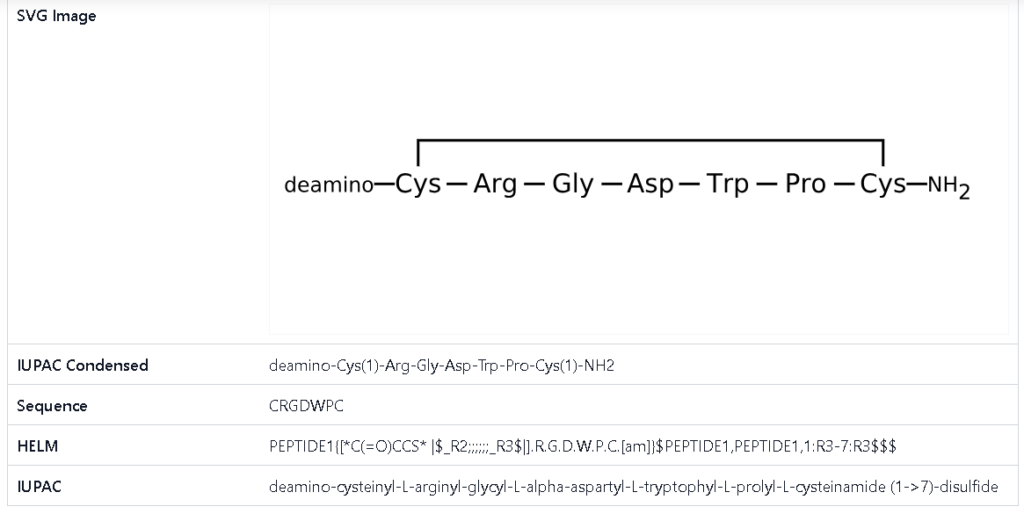
Bevifibatide
Cas 710312-77-9
817.9 g/mol, C34H47N11O9S2
2-[(3S,6S,12S,20R,23S)-20-carbamoyl-12-[3-(diaminomethylideneamino)propyl]-3-(1H-indol-3-ylmethyl)-2,5,8,11,14,22-hexaoxo-17,18-dithia-1,4,7,10,13,21-hexazabicyclo[21.3.0]hexacosan-6-yl]acetic acid
APPROVALS 2025, CHINA 2025, Bio-Thera Solutions, Beitaning RegisteredAcute coronary syndromes
BAT-2094 | batifiban | Beitaning | Betagrin® | Compound I [CN101085809A]
- OriginatorBio-Thera Solutions
- ClassAntiplatelets; Cardiovascular therapies; Cyclic peptides
- Mechanism of ActionIntegrin alphaVbeta3 antagonists; Platelet glycoprotein GPIIb-IIIa complex anatgonists
- 02 Dec 2024Zhujiang Hospital plans a phase II BCAIS-I trial for Acute ischemic stroke in China (IV) (NCT06712004)
- 07 Aug 2024Chemical structure information updated
- 28 Jun 2024Registered for Acute coronary syndromes in China (IV) – First global approval
- Correctin
- 7AKM76YKN5
Bevifibatide is a synthetic cyclic heptapeptide, and its synthesis involves several stages of peptide chemistry. The primary methods used for producing peptides of this nature are solid-phase peptide synthesis (SPPS), followed by cleavage, purification, and cyclization.
Bevifibatide is a cyclic peptide with the Peptide sequence Arg-Gly-Asp-MeAsp-Phg-Val-Nal.
Bevifibatide (Bio-Thera Solutions) is a synthetic cyclic heptapeptide that functions as a αIIbβ3 and αvβ3 integrin receptor antagonist [1]. It was designed to inhibit platelet aggregation as an antiplatelet cardiovascular therapy.
SYN
CN101085809
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN83278873&_cid=P21-MEUT2B-21989-1
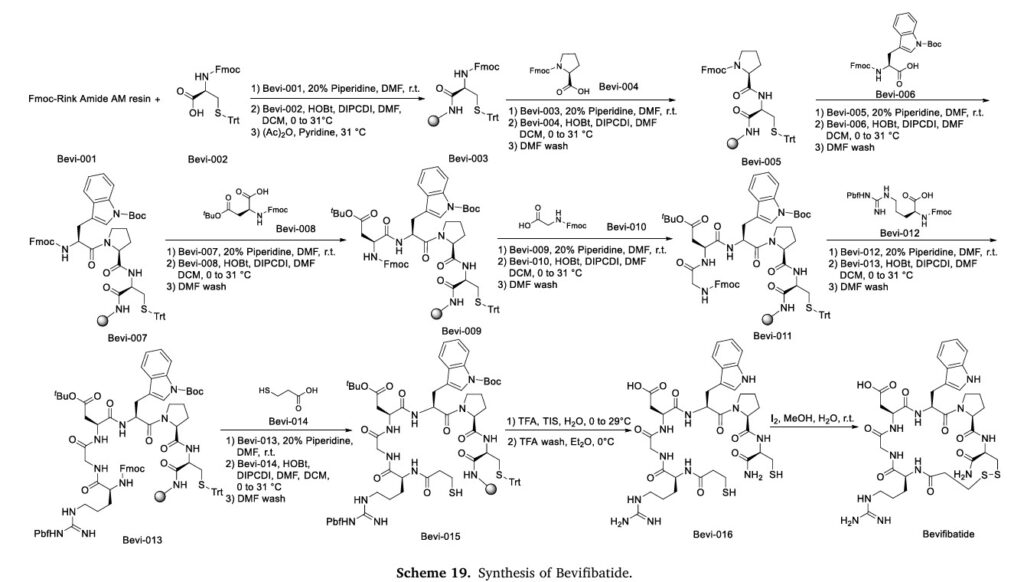
| Example 1: Fmoc solid phase synthesis |
| 1: Synthesis of Fmoc-Cys(Trt)-HN-Rink Amide AM resin |
| (1) Fmoc-Rink Amide AM resin (produced by Tianjin Nankai Hecheng Technology Co., Ltd., substitution degree 0.59 mmol/g, 8.4746 g) was added to the solid phase reaction column, washed three times with DMF, and swelled with DCM for 30 minutes. |
| (2) The solution was drained and Fmoc-protection was removed with 20% piperidine in DMF at room temperature for 20 minutes. |
| (3) The solution was drained, the resin was washed five times with DMF, and the solution was drained. |
| (4) Fmoc-Cys(Trt)-OH (2.9285 g), HOBt (0.6755 g), and DIPCDI (0.8 ml) were dissolved in DMF (20 ml) and DCM (20 ml) and pre-reacted in an ice bath for 20 minutes. |
| (5) Add the above reaction solution to the solid phase reaction column, N 2 Stir with air flow to ensure full contact and reaction with the resin, and react at room temperature (31°C) for 2 hours. |
| (6) The solution was drained, and the resin was washed three times with DMF and once with DCM. Acetic anhydride (10 ml), pyridine (8 ml), and N 2 Stir with air flow to ensure full contact and reaction with the resin, and react at room temperature (31°C) for 10 hours. |
| (7) The solution was drained, and the resin was washed three times with DMF, three times with DCM, and three times with methanol. The resin was then dried under vacuum to obtain Fmoc-Cys(Trt)-HN-Rink Amide AM resin (10.2578 g). The degree of substitution was measured to be 0.5086 mmol/g. |
| 2: Synthesis of Fmoc-Pro-Cys(Trt)-HN-Rink Amide AM resin |
| (1) 10.2578 g of Fmoc-Cys(Trt)-Rink Amide AM resin (substitution degree: 0.5086 mmol/g) was added to the solid phase reaction column, washed three times with DMF, and swelled with DCM for 30 minutes. |
| (2) The solution was drained and Fmoc-protection was removed with 20% piperidine in DMF at room temperature for 20 minutes. |
| (3) The solution was drained, the resin was washed five times with DMF, and the solution was drained. |
| (4) Fmoc-Pro-OH (5.061 g), HOBt (3.04 g), and DIPCDI (7.5 ml) were dissolved in DMF (20 ml) and DCM (20 ml) and pre-reacted in an ice bath for 20 minutes. |
| (5) Add the above reaction solution to the solid phase reaction column, N 2 Stir with air flow to ensure full contact and reaction with the resin. React at room temperature (30°C) for 2 hours, and monitor the reaction progress with Kaiser test. |
| (6) The solution was drained and the resin was washed three times with DMF to obtain Fmoc-Pro-Cys(Trt)-HN-Rink Amide AM resin. |
| 3: Synthesis of Mpr-X-Gly-Asp(OtBu)-Trp(Boc)-Pro-Cys(Trt)-HN-Rink Amide AM resin, where X is Arg(Pbf), Har(Pbf) or Lys(Boc) |
| The reaction steps for coupling each protected amino acid are the same as 2, except that the protected amino acids to be coupled are: Fmoc-Trp(Boc)-OH (7.899 g); Fmoc-Asp(OtBu)-OH (6.172 g); Fmoc-Gly-OH (4.460 g); Fmoc-X-OH (9.732 g); Mpr (1.592 g). |
| 4: Linear crude peptide Mpr-X-Gly-Asp-Trp-Pro-Cys-NH 2 Preparation |
| (1) The resin obtained in step 3 was washed three times with DMF, three times with DCM, and three times with methanol, and then dried under vacuum to obtain 21.182 g of Mpr-X-Gly-Asp(OtBu)-Trp(Boc)-Pro-Cys(Trt)-HN-Rink Amide AM resin. |
| (2) The obtained resin was placed in a round-bottom flask and TFA (180 ml), H 2 A mixed solution of O (10 ml) and TIS (10 ml) was introduced into 2 , stir electromagnetically in an ice bath for 10 minutes, remove the ice bath, and react at room temperature (29°C) for 2 hours. |
| (3) After the reaction is completed, the solution is filtered, and the resin is washed twice with TFA. The filtrates are combined and ice-cold ether (2 L) is added to the filtrate. A white precipitate is precipitated, and the precipitate is collected by centrifugation and fully dried in vacuo. |
| (4) The dried white precipitate (4.237 g) was collected to obtain the crude linear peptide Mpr-X-Gly-Asp-Trp-Pro-Cys-NH 2 , sealed and stored at -20℃. |
| 5: Linear crude peptide Mpr-X-Gly-Asp-Trp-Pro-Cys-NH 2 Cyclization |
| The crude linear peptide of batifiban and its analogues obtained in Example 4 was dissolved in water, and 1 mmol/ml of I 2 The mixture was stirred for 30 minutes at room temperature, and the cyclization reaction was followed by analytical HPLC until completion, thereby obtaining Mpr-X-Gly-Asp-Trp-Pro-Cys-NH 2 (Disulfide bridge,Mpr1-Cys7)。 |
SYN
European Journal of Medicinal Chemistry 291 (2025) 117643
Bevifibatide, developed by Bio-Thera Solutions, is a synthetic peptide that functions as a glycoprotein IIb/IIIa (GP IIb/IIIa) receptor antagonist. It is marketed under the brand name Beitaning. In 2024, the
NMPA approved Bevifibatide citrate injection for use in patients with acute coronary syndrome undergoing percutaneous coronary intervention (PCI), including coronary stent implantation, to reduce the risk of acute occlusion, in-stent thrombosis, no-reflow, and slow flow phenomena. Bevifibatide exerts its therapeutic effects by specifically binding to the GP IIb/IIIa receptors on platelets, thereby inhibiting the binding of fibrinogen, von Willebrand factor, and other adhesive ligands to these receptors [80]. This inhibition prevents platelet aggregation, reducing the risk of thrombotic complications during and after PCI procedures. The clinical efficacy of Bevifibatide was demonstrated in a multicenter Phase III trial involving patients with acute coronary syndrome undergoing PCI (NCT04567890). The study achieved its primary efficacy endpoint, with the composite endpoint event rate at 30 days post-procedure being significantly lower in the Bevifibatide group (4.06%) compared to the control group (6.56 %), indicating superior antithrombotic efficacy. Regarding toxicity, Bevifibatide was generally well-tolerated. The most common adverse events included bleeding complications, which are consistent with the pharmacological action of GP IIb/IIIa inhibitors. These events were manageable with appropriate clinical interventions, and the overall safety profile was comparable to other agents in its class. The approval of Bevifibatide provides a new therapeutic option for patients undergoing PCI, aiming to enhance procedural safety by mitigating thrombotic risks associated with such
interventions [81].
The synthetic route of Bevifibatide, shown in Scheme 19, comprises sequential amidation reactions: Bevi-001 reacts with Bevi-002 to form Bevi-003, which undergoes deprotection and subsequent coupling with
Bevi-004 to generate Bevi-005 [82]. This intermediate undergoes consecutive amidation steps with Bevi-006 and Bevi-008, producing Bevi-007 and Bevi-009 respectively. Bevi-009 then reacts with Bevi-010
to form Bevi-011, followed by coupling with Bevi-012 to yield Bevi-013. Subsequent amidation with Bevi-014 produces Bevi-015, which undergoes TFA-mediated deprotection to give Bevi-016. The final synthesis involves oxidation of the sulfhydryl group in Bevi-016 followed by iodine-mediated coupling to afford Bevifibatide.
80-82
[80] G. Tonin, J. Klen, Eptifibatide, an older therapeutic peptide with new indications:
from clinical pharmacology to everyday clinical practice, Int. J. Mol. Sci. 24 (2023)5446.
[81] H. Patel, I. Lunn, S. Hameed, M. Khan, F.M. Siddiqui, A. Borhani, A. Majid, S.M. Bell, M. Wasay, Treatment of cerebral venous thrombosis: a review, Curr. Med.Res. Opin. 40 (2024) 2223–2236.
[82] S. Tan, Y. Yang, Y. Li, Synthesis of N2-(3-mercapto-1-oxopropyl)-L-arginylglycyl-L-α-aspartyl-L
α-tryptophyl-Lα-prolyl-L Its Analogues, 2007 CN101085809A.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
1. Zhao X, Yuan L, Gong Z, Li M, Yuan Y, Geng J. (2025)
New drugs approved by the NMPA in 2024: Synthesis and clinical applications.
Eur J Med Chem, 291: 117643. [PMID:40262297]
- New drugs approved by the NMPA in 2024: Synthesis and clinical applicationsPublication Name: European Journal of Medicinal ChemistryPublication Date: 2025-07-05PMID: 40262297DOI: 10.1016/j.ejmech.2025.117643
- Pharmacokinetic and pharmacodynamic properties of batifiban coadministered with antithrombin agents in Chinese healthy volunteersPublication Name: Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen banPublication Date: 2013-10-20PMID: 24142738DOI: 10.1007/s11596-013-1198-4
- Safety, pharmacokinetic and pharmacodynamic studies of batifiban injection following single- and multiple-dose administration to healthy Chinese subjectsPublication Name: Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen banPublication Date: 2009-02-18PMID: 19224155DOI: 10.1007/s11596-009-0103-7
////////Bevifibatide, APPROVALS 2025, CHINA 2025, Bio-Thera Solutions, Beitaning, BAT 2094, batifiban, 710312-77-9, Correctin, 7AKM76YKN5














