Bosmolisib
CAS 2055765-77-8
MF 2055765-77-8 MW478.3 g/mol
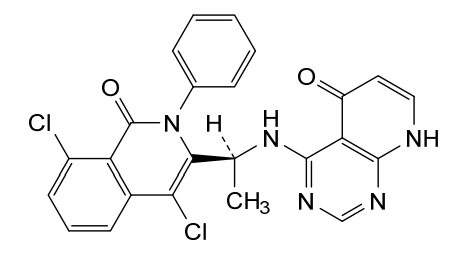
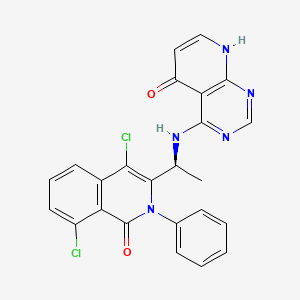
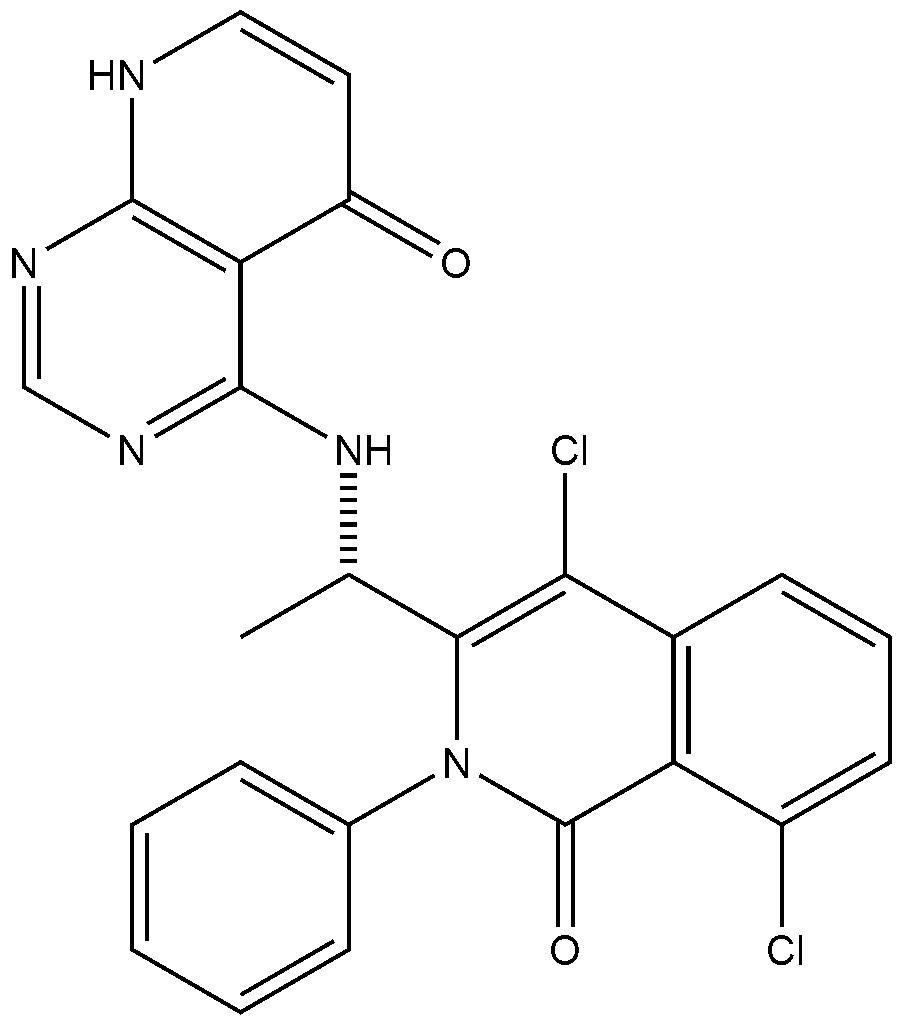
4-{[(1S)-1-(4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl]amino}pyrido[2,3-d]pyrimidin-5(8H)-one
4-[[(1S)-1-(4,8-dichloro-1-oxo-2-phenylisoquinolin-3-yl)ethyl]amino]-8H-pyrido[2,3-d]pyrimidin-5-one
phosphatidylinositol 3-kinase (PI3K) inhibitor, antineoplastic, BR 101801, FJ5CTS1VNJ
- A Study of Bosmolisib (BR101801) in Participants With R/R PTCL.CTID: NCT07180771Phase: Phase 2Status: Not yet recruitingDate: 2025-09-18
- BR101801 in Adult Patients With Advanced Hematologic Malignancies(Phase I)CTID: NCT04018248Phase: Phase 1Status: CompletedDate: 2025-09-10
Bosmolisib is an orally bioavailable inhibitor of phosphoinositide 3-kinase delta (PI3-kinase subunit delta; PI3K-delta; PI3Kdelta) and DNA-dependent protein kinase (DNA-PK), with potential antineoplastic and immunomodulating activities. Upon oral administration, bosmolisib inhibits the activity of both PI3K-delta and DNA-PK. This prevents PI3K-mediated signaling pathways and may lead to the inhibition of cancer cell growth in PI3K-overexpressing tumor cells. Specifically, since PI3K regulates c-myc expression, inhibition of PI3K signaling may lead to a decrease in proliferation of c-myc-expressing tumor cells. Also, by inhibiting the activity of DNA-PK, bosmolisib interferes with the non-homologous end joining (NHEJ) process and prevents the repair of DNA double strand breaks (DSBs) caused by ionizing radiation or chemotherapeutic treatment. This increases chemo- and radiotherapy cytotoxicity by inhibiting the ability of tumor cells to repair damaged DNA. The PI3K pathway is upregulated in a variety of tumors and plays an important role in regulating cancer cell proliferation, growth, and survival. DNA-PK is activated upon DNA damage and plays a key role in repairing double-stranded DNA breaks. The enhanced ability of tumor cells to repair DSBs plays a major role in the resistance of tumor cells to chemo- and radiotherapy. In addition, bosmolisib is able to decrease Tregs and increase CD8 lymphocytes.
- OriginatorBoryung Pharmaceutical
- ClassAntineoplastics; Small molecules
- Mechanism of ActionDNA-activated protein kinase inhibitors; Phosphatidylinositol 3 kinase delta inhibitors; Phosphatidylinositol 3 kinase gamma inhibitors
- Phase IHaematological malignancies
- PreclinicalColorectal cancer
- 18 Sep 2025Boryung Pharmaceutical plans a phase II trial for Peripheral T Cell Lymphoma and Nodal T-follicular helper cell lymphoma (Second-line therapy or greater) in September 2025 (PO, Capsule) (NCT07180771)
- 06 Jan 2025Chemical structure information added.
- 09 Dec 2023Updated efficacy and adverse event data from a phase I trial in Hematological malignancies presented at the 65th American Society of Hematology Annual Meeting and Exposition (ASH-2023
SYN
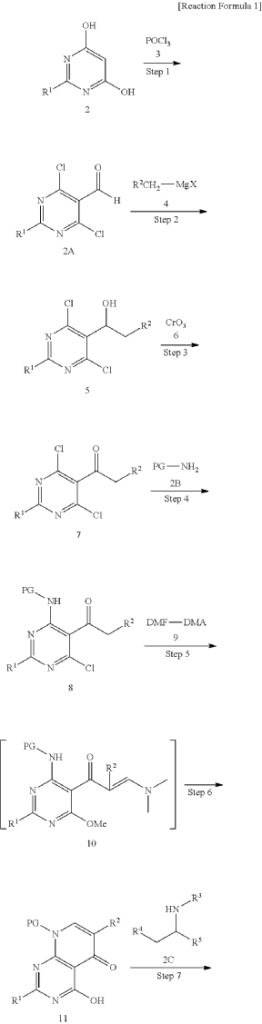
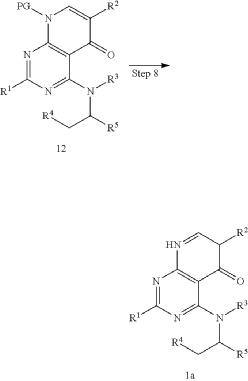
WO 2016/204429.
SYN
xample 1. Preparation of (S)-4-((1-(4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)amino)pyrido[2,3-d]pyrimidin-5(8H)-one
[116](S)-4-((1-(4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)amino)pyrido[2,3-d]pyrimidin-5(8H)-one (4-((1-(4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinolin -3-yl)ethyl)amino)pyrido[2,3-d]pyrimidin-5(8H)-one) represented by the chemical formula 3 above was prepared by the same method as that described in Example 10 of International Patent Publication No.
WO 2016/204429.
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016204429&_cid=P22-MK6A2W-95428-1
<Example 10> Preparation of (S)-4-((l-(4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)amino)pyrido [2,3-d]pyrimidin-5(8H)-one
In Example 5, 50 mg (0.113 vol) of (S)-4— ((1-(8-chloro-1—oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)amino)pyrido [2, 3-d]pyrimidin-5(8H)-one prepared was dissolved in 2 mL of acetic acid, and then 17 mg (0.124 vol) of N—chlorosuccinimide (NCS) was added. The mixture was stirred at 50 ° C for 15 hours, filtered under reduced pressure, neutralized using an aqueous sodium bicarbonate solution, and then the organic layer extracted by adding dichloromethane and water was dried (Na 2 SO 4 ), filtered, concentrated under reduced pressure, and separated by column chromatography (SiO 2 , eluent: dichloromethane/methanol, 30/1 -> dichloromethane/methanol, 10/1) to afford 25 mg (0.052 mmol, 46% yield) of compound (S)— 4-((1— (4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)amino)pyrido[2, 3-d]pyramidin-5(8H)-one as a pale yellow solid.
LH NMR (300 MHz, CDC13) δ 10.99 (d, J = 4.8 Hz, 1Ή), 8.25 (s, 1H) , 7.95(dd, JJ = 1.9 Hz, J = 7.5 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H) , 7.46-7.62 (m, 6H), 7.20 (d, J = 6.7 Hz, 1H) , 6.3 (d, J = 7.5 Hz, 1H), 5.04 (t , J = 67.2 Hz, 1H) , 1.67 (d, J = 7.2 Hz, 3H) .
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US214732247&_cid=P22-MK69S5-86256-1
Example 10: Preparation of (S)-4-((1-(4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinoline-3-yl)ethyl)amino)pyrido[2,3-d]pyrimidine-5(8H)-one
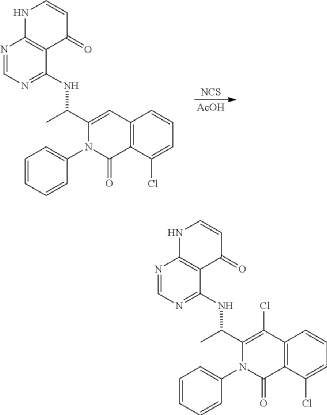
| 50 mg (0.113 mmol) of (S)-4-((1-(8-chloro-1-oxo-2-phenyl-1,2-dihydroisoquinoline-3-yl)ethyl)amino)pyrido[2,3-d]pyrimidine-5(8H)-one prepared in Example 5 was dissolved in 2 mL of acetic acid, to which 17 mg (0.124 mmol) of N-chlorosuccinimide (NCS) was added, followed by stirring at 50° C. for 15 hours. The reaction mixture was filtered under reduced pressure. Saturated sodiumbicarbonate aqueous solution was added thereto, followed by neutralization. Dichloromethane and water were added thereto, followed by extraction. The extracted organic layer was dried (Na 2SO 4), filtered, and concentrated under reduced pressure. The residue was separated by column chromatography (SiO 2, eluent: dichloromethane/methanol, 30/1→dichloromethane/methanol, 10/1) to give 25 mg of the target compound (S)-4-((1-(4,8-dichloro-1-oxo-2-phenyl-1,2-dihydroisoquinoline-3-yl)ethyl)amino)pyrido[2,3-d]pyrimidine-5(8H)-one as a pale yellow solid (0.052 mmol, yield: 46%). |
PAT
- A pharmaceutical composition for preventing or treating a heteroaryl derivative or a pharmaceutically acceptable salt thereof, a method for producing the same, and a PI3 kinase-related disease containing the heteroaryl derivative as an active ingredient.Publication Number: JP-6808905-B2Priority Date: 2015-06-18Grant Date: 2021-01-06
- Heteroaryl derivative or pharmaceutically acceptable salt thereof, method of preparation thereof and pharmaceutical composition to prevent or treat diseases associated with PI3 kinases, which contains the same as active principlePublication Number: ES-2816050-T3Priority Date: 2015-06-18Grant Date: 2021-03-31
- Heteroaryl derivative or pharmaceutically acceptable salt thereof, preparation method therefor, and pharmaceutical compostion for preventing or treating diseases associated with pi3 kinases, containing same as active ingredientPublication Number: US-2018105527-A1Priority Date: 2015-06-18
- Heteroaryl derivative or pharmaceutically acceptable salt thereof, preparation method therefor, and pharmaceutical composition for preventing or treating diseases associated with pi3 kinases, containing same as active ingredientPublication Number: EP-3312175-B1Priority Date: 2015-06-18Grant Date: 2020-07-22
- Heteroaryl derivatives or pharmaceutically acceptable salts thereof, preparation method thereof and pharmaceutical composition for use in preventing or treating pi3 kinase related diseasesPublication Number: TW-I616446-BPriority Date: 2015-06-18Grant Date: 2018-03-01
- HETEROARYL DERIVATIVES OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, PROCESS FOR PRODUCING THE SAME, AND PHARMACEUTICAL COMPOSITIONS FOR PREVENTING OR TREATING PI3-KINASE RELATED DISEASES COMPRISING THE SAME AS THE ACTIVE INGREDIENTPublication Number: JP-2018522852-APriority Date: 2015-06-18
- Heteroaryl derivative or pharmaceutically acceptable salt thereof, preparation method therefor, and pharmaceutical composition for preventing or treating diseases associated with PI3 kinases, containing same as active ingredientPublication Number: US-10526337-B2Priority Date: 2015-06-18Grant Date: 2020-01-07
- Heteroaryl derivative or a pharmaceutically acceptable salt thereof, a method for production thereof and a pharmaceutical composition for preventing or treating diseases associated with pi3 kinases, containing said active substancePublication Number: RU-2719367-C2Priority Date: 2015-06-18Grant Date: 2020-04-17
- Heteroaryl derivative or pharmaceutically acceptable salt thereof, preparation method thereof, and pharmaceutical composition comprising same as active ingredient for preventing or treating PI3 kinase-associated diseasesPublication Number: CN-107690433-APriority Date: 2015-06-18



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
- PI3Kδ/γ inhibitor BR101801 extrinsically potentiates effector CD8+ T cell-dependent antitumor immunity and abscopal effect after local irradiationPublication Name: Journal for ImmunoTherapy of CancerPublication Date: 2022-03PMCID: PMC8921929PMID: 35288465DOI: 10.1136/jitc-2021-003762
- Synergistic radiosensitizing effect of BR101801, a specific DNA-dependent protein kinase inhibitor, in various human solid cancer cells and xenograftsPublication Name: American journal of cancer researchPublication Date: 2021PMCID: PMC8640799PMID: 34873471
/////////bosmolisib, phosphatidylinositol 3-kinase (PI3K) inhibitor, antineoplastic, BR 101801, FJ5CTS1VNJ














