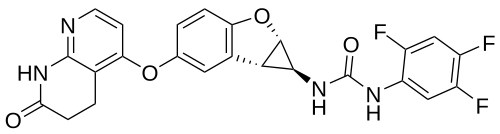
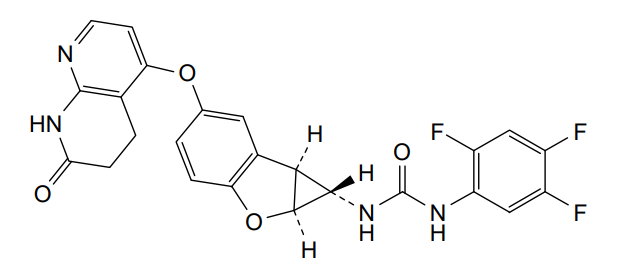
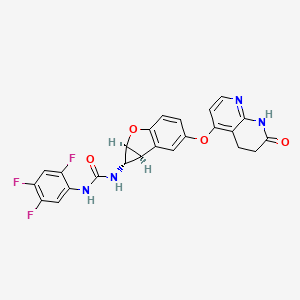
Brimarafenib
CAS 1643326-82-2
MF C24H17F3N4O4 MW482.4 g/mol
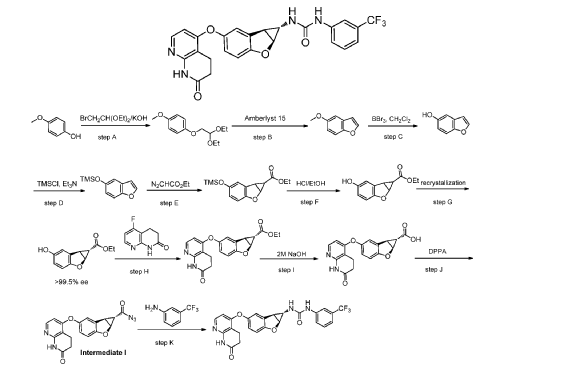
N-{(1S,1aS,6bS)-5-[(7-oxo-5,6,7,8-tetrahydro-1,8-naphthyridin-4-yl)oxy]-1a,6b-dihydro-1H-cyclopropa[b]benzofuran-1-yl}-N′-(2,4,5-trifluorophenyl)urea
rapidly accelerated fibrosarcoma (Raf) kinase inhibitor,
- 1-((1S,1aS,6bS)-5-((7-oxo-6,8-dihydro-5H-1,8-naphthyridin-4-yl)oxy)-1a,6b-dihydro-1H-cyclopropa(b)(1)benzofuran-1-yl)-3-(2,4,5-trifluorophenyl)urea
- 1-[(1S,1aS,6bS)-5-[(7-oxo-6,8-dihydro-5H-1,8-naphthyridin-4-yl)oxy]-1a,6b-dihydro-1H-cyclopropa[b][1]benzofuran-1-yl]-3-(2,4,5-trifluorophenyl)urea
Antineoplastic, MapKure, LLC, SpringWorks Therapeutics, BeiGene, BGB-3245, BGB 3245, GXS33OY2CB
Brimarafenib is an investigational new drug that is being evaluated for the treatment of cancer. It targets the proto-oncogene BRAF with activating mutations BRAF mutations (such as V600E), non-V600 BRAF mutations, and RAF fusions.[1][2]
It is being developed by MapKure, LLC, a joint venture between SpringWorks Therapeutics and BeiGene.[1]
Brimarafenib is an orally available inhibitor of both monomer and dimer forms of activating mutations of the serine/threonine-protein kinase BRAF (B-raf) protein, including V600 BRAF mutations, non-V600 BRAF mutations, and RAF fusions, with potential antineoplastic activity. Upon administration, brimarafenib targets and binds to both monomeric and dimeric forms of activating BRAF mutations and fusions. This may result in the inhibition of BRAF-mediated signaling and inhibit proliferation in tumor cells expressing BRAF mutations and fusions. BRAF belongs to the RAF family of serine/threonine protein kinases and plays a role in regulating the mitogen-activated protein kinase (MAPK)/ extracellular signal-regulated kinase (ERK) signaling pathway, which is often dysregulated in human cancers and plays a key role in tumor cell proliferation and survival. BRAF mutations and fusions have been identified in a number of solid tumors and are drivers of cancer growth.
PAT
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014206343&_cid=P22-MG0802-32937-1

PAT
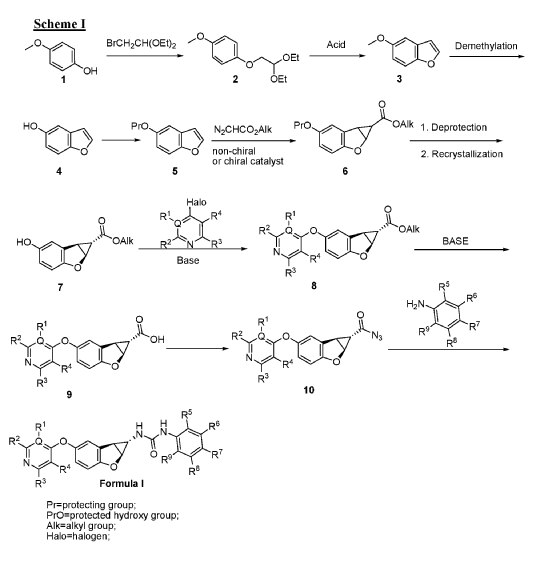
Fused tricyclic urea compounds as raf kinase and/or raf kinase dimer inhibitors
Publication Number: WO-2014206343-A1
Priority Date: 2013-06-28
- Fused tricyclic urea compounds as raf kinase and/or raf kinase dimer inhibitorsPublication Number: US-2016368914-A1Priority Date: 2013-06-28
- Fused tricyclic urea compounds as raf kinase and/or raf kinase dimer inhibitorsPublication Number: US-2017233391-A1Priority Date: 2013-06-28
- Fused tricyclic urea compounds as raf kinase and/or raf kinase dimer inhibitorsPublication Number: US-2019144446-A1Priority Date: 2013-06-28
- Fused tricyclic urea compounds as Raf kinase and/or Raf kinase dimer inhibitorsPublication Number: US-9670203-B2Priority Date: 2013-06-28Grant Date: 2017-06-06
- Fused tricyclic urea compounds as raf kinase and/or raf kinase dimer inhibitorsPublication Number: US-9920055-B2Priority Date: 2013-06-28Grant Date: 2018-03-20



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | BGB-3245 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1643326-82-2 |
| PubChem CID | 117807031 |
| IUPHAR/BPS | 13203 |
| ChemSpider | 129144353 |
| UNII | GXS33OY2CB |
| Chemical and physical data | |
| Formula | C24H17F3N4O4 |
| Molar mass | 482.419 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- “Brimarafenib”.
- Tellenbach FL, Seiler LL, Johnson M, Rehrauer H, Schukla P, Martinez-Gomez J, et al. “Combination of the Novel Raf Dimer Inhibitor Brimarafenib with the Mek Inhibitor Mirdametinib is Effective Against Nras Mutant Melanoma”. SSRN: 4934723. doi:10.2139/ssrn.4934723.
///////Brimarafenib, Antineoplastic, MapKure, LLC, SpringWorks Therapeutics, BeiGene, BGB-3245, BGB 3245, GXS33OY2CB














