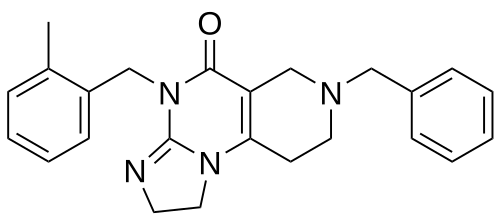
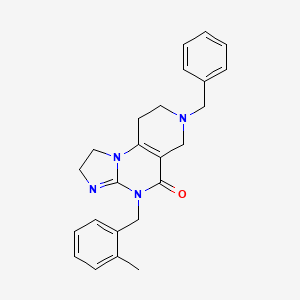
Dordaviprone
WeightAverage: 386.499
Monoisotopic: 386.210661473
Chemical FormulaC24H26N4O
- TIC10
- CAS 1616632-77-9
- Dordaviprone
- ONC201
- ONC 201
- 9U35A31JAI
- NSC-350625
11-benzyl-7-[(2-methylphenyl)methyl]-2,5,7,11-tetrazatricyclo[7.4.0.02,6]trideca-1(9),5-dien-8-one
Product Ingredients
| Ingredient | UNII | CAS | InChI Key |
|---|---|---|---|
| Dordaviprone dihydrochloride | 53VG71J90J | 1638178-82-1 | Not applicable |
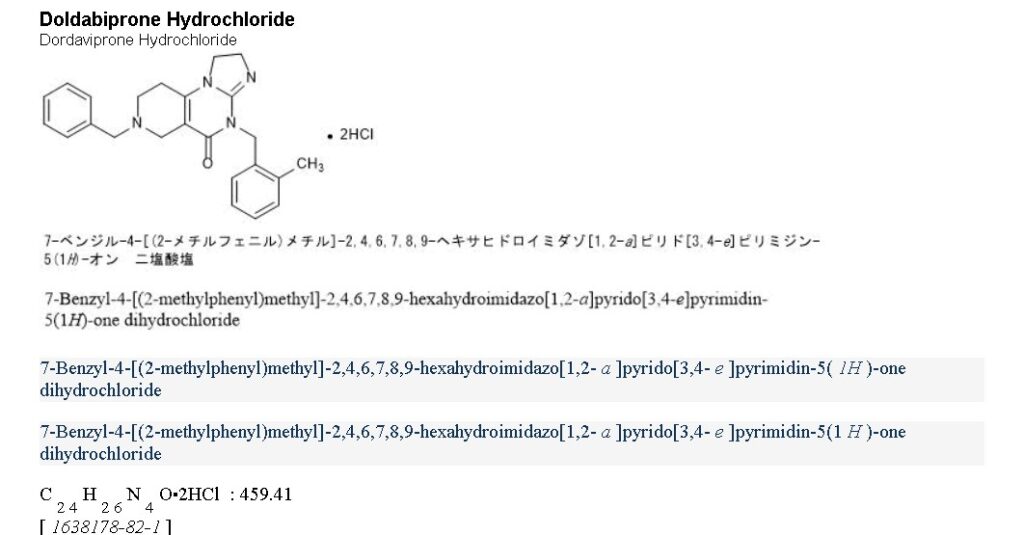
- 11-benzyl-7-(o-tolylmethyl)-2,5,7,11-tetrazatricyclo[7.4.0.02,6]trideca-1(9),5-dien-8-one
- 11-benzyl-7-[(2-methylphenyl)methyl]-2,5,7,11-tetrazatricyclo[7.4.0.0(2,6)]trideca-1(9),5-dien-8-one
- 7-Benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-e]pyrimidin-5(1H)-one
- 7-benzyl-4-[(2-methylphenyl)methyl]-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-e]pyrimidin-5(1H)-one
FDA 8/6/2025, Modeyso, To treat diffuse midline glioma harboring an H3 K27M mutation with progressive disease following prior therapy
Dordaviprone, sold under the brand name Modeyso is an anti-cancer medication used for the treatment of diffuse midline glioma (a type of brain tumor).[1][2] Dordaviprone is a protease activator of the mitochondrial caseinolytic protease P.[1] It is dopamine receptor D2 antagonist and an allosteric activator of the mitochondrial caseinolytic protease P.[3]
Dordaviprone was approved for medical use in the United States in August 2025.[2] It is the first approval of a systemic therapy for H3 K27M-mutant diffuse midline glioma by the US Food and Drug Administration.[2]
Dordaviprone is an organic heterotricyclic compound that is 2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-e]pyrimidin-5(1H)-one substituted by 2-methylbenzyl and benzyl groups at positions 4 and 7, respectively. It is a selective antagonist of the dopamine receptor D2 and an allosteric agonist of mitochondrial protease caseinolytic protease P. It has a role as an antineoplastic agent, a dopamine receptor D2 antagonist and an apoptosis inducer. It is a member of toluenes, a member of benzenes and an organic heterotricyclic compound.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- https://pp.jazzpharma.com/pi/modeyso.en.USPI.pdf [bare URL PDF]
- “FDA grants accelerated approval to dordaviprone for diffuse midline glioma”. U.S. Food and Drug Administration (FDA). 6 August 2025. Retrieved 7 August 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Prabhu VV, Morrow S, Rahman Kawakibi A, Zhou L, Ralff M, Ray J, et al. (December 2020). “ONC201 and imipridones: Anti-cancer compounds with clinical efficacy”. Neoplasia. 22 (12). New York, N.Y.: 725–744. doi:10.1016/j.neo.2020.09.005. PMC 7588802. PMID 33142238.
- “Jazz Pharmaceuticals Announces U.S. FDA Approval of Modeyso (dordaviprone) as the First and Only Treatment for Recurrent H3 K27M-mutant Diffuse Midline Glioma” (Press release). Jazz Pharmaceuticals. 6 August 2025. Retrieved 10 August 2025 – via PR Newswire.
- World Health Organization (2023). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 89”. WHO Drug Information. 37 (1). hdl:10665/366661.
External links
- Clinical trial number NCT02525692 for “Oral ONC201 in Adult Recurrent Glioblastoma” at ClinicalTrials.gov
- Clinical trial number NCT03295396 for “ONC201 in Adults With Recurrent H3 K27M-mutant Glioma” at ClinicalTrials.gov
- Clinical trial number NCT03416530 for “ONC201 in Pediatric H3 K27M Gliomas” at ClinicalTrials.gov
- Clinical trial number NCT05392374 for “Expanded Access Use of ONC201 in a Patient With Diffuse Intrinsic Pontine Gliomas” at ClinicalTrials.gov
- Clinical trial number NCT03134131 for “Expanded Access to ONC201 for Patients With H3 K27M-mutant and/or Midline High Grade Gliomas” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Trade names | Modeyso |
| Other names | ONC201, ONC-201 |
| AHFS/Drugs.com | Modeyso |
| License data | US DailyMed: Dordaviprone |
| Routes of administration | By mouth |
| Drug class | Protease activator |
| ATC code | None |
| Legal status | |
| Legal status | US: ℞-only[1] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1616632-77-9as HCl: 1638178-82-1 |
| PubChem CID | 73777259 |
| DrugBank | DB14844as HCl: DBSALT003291 |
| ChemSpider | 30904994 |
| UNII | 9U35A31JAIas HCl: 53VG71J90J |
| KEGG | D12733as HCl: D12734 |
| ChEBI | CHEBI:232328 |
| ChEMBL | ChEMBL4297310 |
| Chemical and physical data | |
| Formula | C24H26N4O |
| Molar mass | 386.499 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
//////Dordaviprone, Modeyso, FDA 2025, APPROVALS 2025, TIC10, 1616632-77-9, Dordaviprone, ONC201, ONC 201, 9U35A31JAI, NSC 350625
Product Ingredients
| Ingredient | UNII | CAS | InChI Key |
|---|---|---|---|
| Dordaviprone dihydrochloride | 53VG71J90J | 1638178-82-1 | Not applicable |














