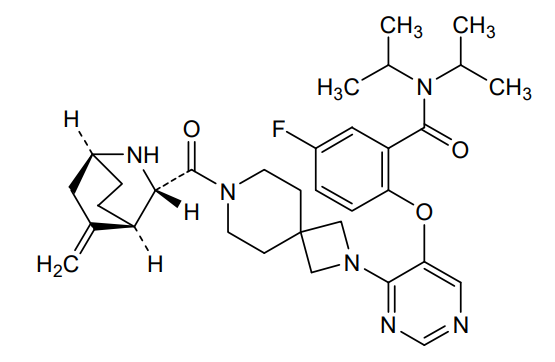
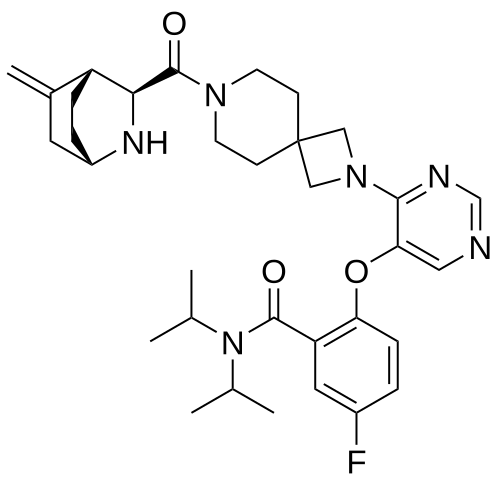
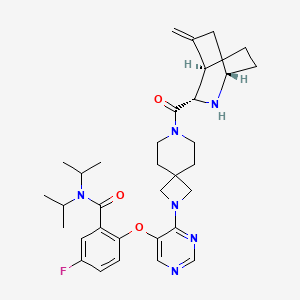
Enzomenib
CAS 2412555-70-3
MF C33H43FN6O3 MW 590.7 g/mol
5-fluoro-2-[4-[7-[(1S,3S,4R)-5-methylidene-2-azabicyclo[2.2.2]octane-3-carbonyl]-2,7-diazaspiro[3.5]nonan-2-yl]pyrimidin-5-yl]oxy-N,N-di(propan-2-yl)benzamide
5-fluoro-2-[(4-{7-[(1S,3S,4R)-5-methylidene-2-azabicyclo[2.2.2]octane-3-carbonyl]-2,7-
diazaspiro[3.5]nonan-2-yl}pyrimidin-5-yl)oxy]-N,Ndi(propan-2-yl)benzamide
menin-MLL (mixed-lineage leukemia) protein, interaction inhibitor, antineoplastic, DSP-5336, Fast Track, Orphan Drug designations
Enzomenib is an investigational new drug that is being evaluated for the treatment of acute leukemia.[1] It is a small molecule inhibitor that targets the interaction between menin and mixed-lineage leukemia (MLL) proteins.[2] Enzomenib particularly in patients with KMT2A (MLL) rearrangements or NPM1 mutations.[3]
The U.S. Food and Drug Administration (FDA) has granted both Fast Track and Orphan Drug designations to Enzomenib.[4]
Enzomenib is an orally bioavailable, small molecule inhibitor of menin, with potential antineoplastic activity. Upon oral administration, enzomenib targets and binds to the nuclear protein menin, thereby preventing the interaction between the two proteins menin and menin-mixed lineage leukemia (MLL; myeloid/lymphoid leukemia; KMT2A) and the formation of the menin-MLL complex. This reduces the expression of downstream target genes and results in an inhibition of the proliferation of MLL-rearranged leukemic cells. The menin-MLL complex plays a key role in the survival, growth, transformation and proliferation of certain kinds of leukemia cells.
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US295244745&_cid=P21-MGISYZ-31333-1
Example 3 to 19
| The following compounds of Examples 3 to 19 were prepared according to a similar method to Example 1 by using each corresponding starting compound. |

PAT
Optically active azabicyclo derivatives
Publication Number: JP-7614262-B2
Priority Date: 2018-08-27
Grant Date: 2025-01-15
- Optically active azabicyclo derivativesPublication Number: CN-112585140-BPriority Date: 2018-08-27Grant Date: 2023-07-04
- Optically active azabicyclo ring derivativePublication Number: JP-2023134729-APriority Date: 2018-08-27
- Chiral azabicyclyl compound derivativePublication Number: TW-I815954-BPriority Date: 2018-08-27Grant Date: 2023-09-21
- Optically active azabicyclo ring derivativePublication Number: US-11911381-B2Priority Date: 2018-08-27Grant Date: 2024-02-27
- Optically active azabicyclo ring derivativePublication Number: US-2024148727-A1Priority Date: 2018-08-27
- Optically active azabicyclic derivativePublication Number: AU-2019327006-A1Priority Date: 2018-08-27
- Optically active azabicyclic derivativePublication Number: EP-3845533-A1Priority Date: 2018-08-27
- Optically active azabicyclo ring derivativePublication Number: US-2021338668-A1Priority Date: 2018-08-27
- Optically active azabicyclo ring derivativePublication Number: US-11369605-B2Priority Date: 2018-08-27Grant Date: 2022-06-28
- Optically active azabicyclo ring derivativePublication Number: US-2022288072-A1Priority Date: 2018-08-27
- Optically active azabicyclo ring derivativePublication Number: US-2020157114-A1Priority Date: 2018-08-27
- Optically active azabicyclic derivativePublication Number: WO-2020045334-A1Priority Date: 2018-08-27
- Optically active azabicyclo ring derivativesPublication Number: JP-2020105191-APriority Date: 2018-08-27
- Chiral azabicyclyl compound derivativePublication Number: TW-202024082-APriority Date: 2018-08-27
- Optically active azabicyclo ring derivativePublication Number: US-10815241-B2Priority Date: 2018-08-27Grant Date: 2020-10-27



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- “Enzomenib – Sumitomo Pharma”. AdisInsight. Springer Nature Switzerland AG.
- Dempke WC, Desole M, Chiusolo P, Sica S, Schmidt-Hieber M (September 2023). “Targeting the undruggable: menin inhibitors ante portas”. Journal of Cancer Research and Clinical Oncology. 149 (11): 9451–9459. doi:10.1007/s00432-023-04752-9. PMC 11798168. PMID 37103568.
- “Sumitomo Pharma Presents New Clinical Data on DSP-5336 at the European Hematology Association 2024 Congress”. Sumitomo Pharma Co., Ltd. 14 June 2024.
- Flaherty C (15 July 2024). “FDA Grants Fast Track Designation to DSP-5336 in KMT2A/NMP1+ AML”. OncLive.
| Clinical data | |
|---|---|
| Other names | DSP-5336 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2412555-70-3 |
| PubChem CID | 146430058 |
| DrugBank | DB18514 |
| ChemSpider | 129534736 |
| UNII | VW83Y2JLZ5 |
| ChEMBL | ChEMBL5314915 |
| Chemical and physical data | |
| Formula | C33H43FN6O3 |
| Molar mass | 590.744 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
//////////enzomenib, Interaction inhibitor, antineoplastic, DSP 5336, Fast Track, Orphan Drug designations














