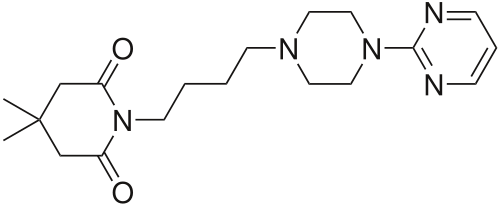
Gepirone
CAS 83928-76-1
BMY 13805, MJ 13805, ORG 13011, Gepirona,
JW5Y7B8Z18
FDA 9/22/2023, Gepirone is indicated for the treatment of major depressive disorder (MDD) in adults
Exxua |
Average: 359.474
Monoisotopic: 359.232125194
Chemical Formula
C19H29N5O2
4,4-dimethyl-1-{4-[4-(pyrimidin-2-yl)piperazin-1-yl]butyl}piperidine-2,6-dione
| Ingredient | UNII | CAS | InChI Key |
|---|---|---|---|
| Gepirone Hydrochloride | 80C9L8EP6V | 83928-66-9 | DGOCVISYYYQFEP-UHFFFAOYSA-N |
Gepirone, sold under the brand name Exxua, is a medication used for the treatment of major depressive disorder.[1] It is taken orally.[1]
Side effects of gepirone include dizziness, nausea, insomnia, abdominal pain, and dyspepsia (indigestion).[1] Gepirone acts as a partial agonist of the serotonin 5-HT1A receptor.[1][2] An active metabolite of gepirone, 1-(2-pyrimidinyl)piperazine, is an α2-adrenergic receptor antagonist.[1][3] Gepirone is a member of the azapirone group of compounds.[2]
Gepirone was synthesized by Bristol-Myers Squibb in 1986 and was developed and marketed by Fabre-Kramer Pharmaceuticals.[4] It was approved for the treatment of major depressive disorder in the United States in September 2023.[4] This came after the drug had been rejected by the Food and Drug Administration (FDA) three times over two decades due to insufficient evidence of effectiveness.[5]
History
Gepirone was developed by Bristol-Myers Squibb in 1986,[5] but was out-licensed to Fabre-Kramer in 1993. The FDA rejected approval for gepirone in 2002 and 2004.[5] It was submitted for the preregistration (NDA) phase again in May 2007 after adding additional information from clinical trials as the FDA required in 2009. However, in 2012 it once again failed to convince the FDA of its qualities for treating anxiety and depression.[5] In December 2015, the FDA once again gave gepirone a negative review for depression due to concerns of efficacy.[12] However, in March 2016, the FDA reversed its decision and gave gepirone ER a positive review.[13] Gepirone ER was finally approved for the treatment of major depressive disorder in the United States in September 2023.[5]
SYN
Synthesis
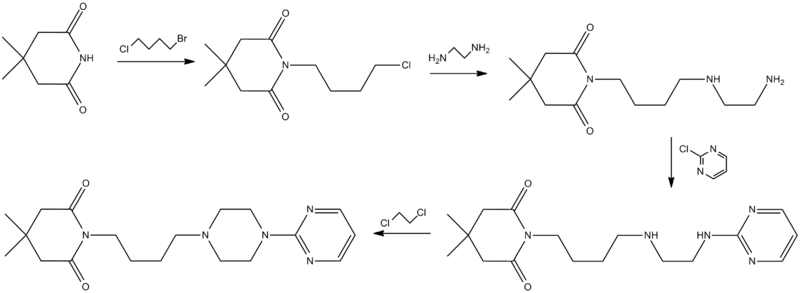
Ormaza, V. A.; 1986, ES 8606333.
SYN
https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/gepirone
Gepirone (Exxua)
Gepirone, a selective and affinitive 5-hydroxytryptamine 1A (5-HT1A) agonist, received FDA approval on September 22, 2023, to treat major depressive disorder in adults [37]. Gepirone, an azapirone compound, is a pharmacological derivative of buspirone that exhibits specific activity on both pre- and post-synaptic 5-HT1A receptors. Despite the promising results observed in previous clinical trials for Gepirone, the need for frequent administration is a requirement due to its formulation as an immediate-release tablet and its short half-lives. Gepirone did not become a potential candidate for a new antidepressant until an extended-release formulation of it was developed [38–40].
An efficient approach of Gepirone has been disclosed in Scheme 11 [41]. Substitution of 2-(piperazin-1-yl)pyrimidine (GEPI-001) with 1,4-dibromobutane (GEPI-002), followed by ring opening and addition, generated the final product Gepirone.
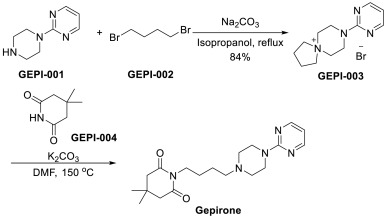
PATENT
https://patents.google.com/patent/WO2020148621A1/en
Gepirone (4,4-dimethyl-l-[4-[4-(2-pyrimidinyl)-l-piperazinyl]butyl]-2,6- piperidindione) is an antidepressant and anxiolytic medicament belonging to the azapirone group, currently at the pre-registration stage in the USA. Like other azapirones, gepirone is a selective partial agonist of the 5-HT1A receptor.
The prior art includes some synthesis strategies for the preparation of gepirone (I); they are mainly multi-step reactions which present various drawbacks such as economic inefficiency, low yield and low industrial applicability.
The synthesis of gepirone (I) is described in J. Med. Chem. 1988, 31, 1967-1971; WO 2012/016569; EP 0680961; Dier Junyi Daxue Xuebao, 26(2), 223-224; 2005; Patentschrift (CH), 682564, 15 October 1993; Heterocycles, 36(7), 1463-9, 1993; and Bioorganic & Medicinal Chemistry Letters, 14(7), 1709-1712, 2004.
The scientific article published in J. Med. Chem. 1988, 31, 1967-1971 describes the synthesis of gepirone (I) from N-bromobutyl phthalimide (2) and l-(pyrimidin-2- yl)piperazine (3) in the presence of potassium carbonate to give the intermediate 2-(4-(4- (pyrimidin-2-yl)piperazin-l-yl)butyl)isoindoline-l,3-dione (4), from which the phthalimide protecting group is removed with hydrazine hydrate. The compound 4-(4-(pyrimidin-2-yl)piperazin- 1 -yl)butan- 1 -amine (5) thus synthesised is used in the reaction with 4,4-dimethyldihydro-2H-pyran-2,6(3H)-dione (6) to obtain gepirone (I) (Scheme 1).
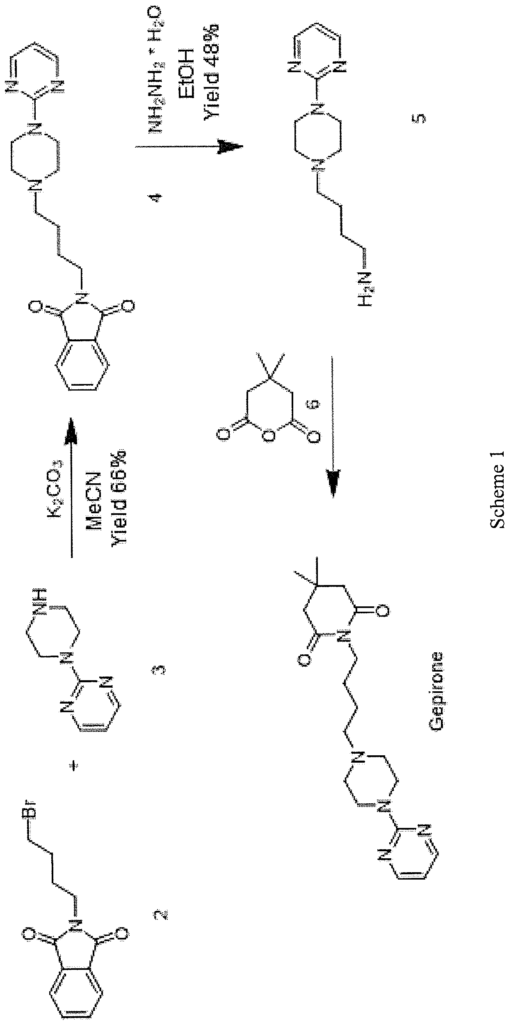
SCHEME 2
In WO 2012/016569, gepirone (I) is synthesised in four synthesis steps from 1- (pyrimidin-2-yl)pipetazine (3) and 4-((tert-butoxycarbonyl)amino)butanoic acid (7) with the use of condensing agents, such as HATU, and strong reducing agents such as lithium aluminium hydride. The use of condensing agents makes the process practically unusable on an industrial scale because of their high economic impact and the formation of countless by-products which are difficult to remove during the work-up step (Scheme 2).
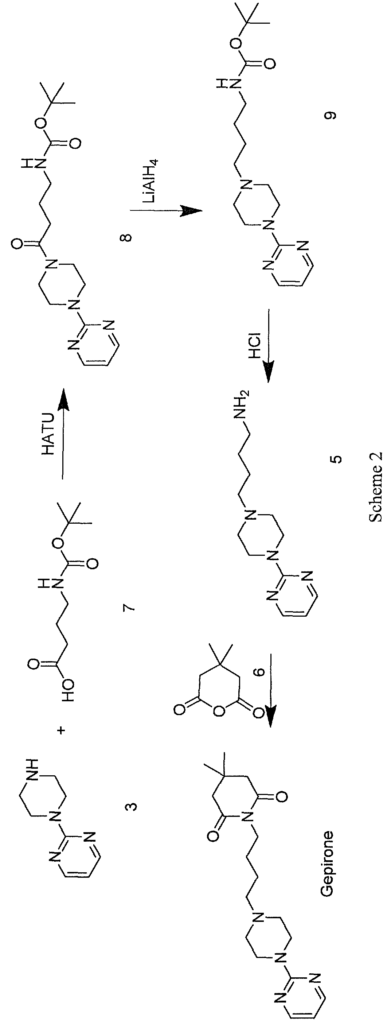
A further approach for the synthesis of gepirone is described in the literature (Ί). This strategy, disclosed in EP 0680961, initially involves synthesising (i) a spiranic intermediate (8-(pyrimidin-2-yl)- 5 ,8-diazaspiro [4,5] decan- 5 -ium bromide) (11) from 1- (pyrimidin-2-yl)piperazine (3) and (ii) 1,4-dibromobutane (10), then opening the spiranic compound (11) with the use of potassium 4,4-dimethyl-2,6-dioxopiperidin-l-ide (13), a secondary amine characterised by a high level of nucleophilicity (Scheme 3)
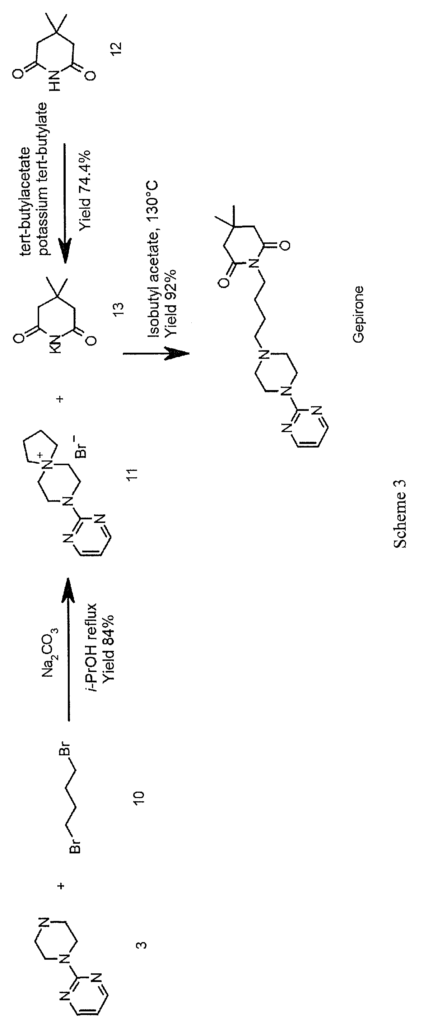
A novel approach to the synthesis of gepirone (I) has now been found, which involves opening spiranic derivative (11) to give 4-(4-(pyrimidin-2-yl)piperazin-l- yl)butan- 1 -amine (5), using suitable nitrogen nucleophile precursors of a primary amino group having the following characteristics: moderate nucleophilicity, so as to prevent reaction by-products, and easy generation of a primary amino group by means of a mild work-up (Scheme 4).
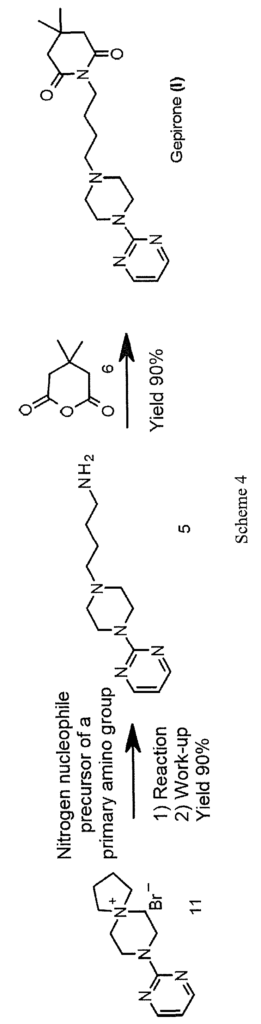
synthesis of gepirone (I) from
8-(pyrimidin-2-yl)-5,8-diazaspiro[4,5]decan-5-ium bromide (11), which is commercially available or easily obtainable by well-known procedures, such as those described in US 4351939.
Spiranic derivative (11) initially undergoes selective opening by suitable nitrogen nucleophile precursors of a primary amino group, such as di-tert-butyl iminodicarboxylate (14) and 2,2,2-trifluoroacetamide (15), in the presence of an organic and/or inorganic base. The opening of spiranic ring (11), followed by a simple, mild acid- base work-up, produces, in a single high-yield synthesis step, the intermediate 4-(4-(pyrimidin-2-yl)piperazin- 1 -yl)butan- 1 -amine (5), which is converted to gepirone (I) by reaction with 4,4-dimethyldihydro-2H-pyran-2,6(3H)-dione (6) (Scheme 5).
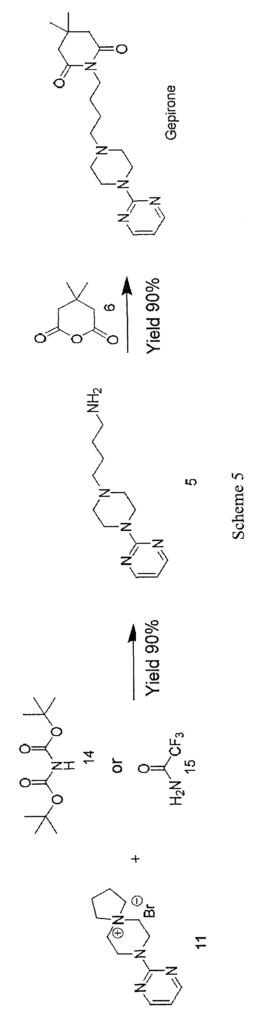
Example 1 – 4-(4-(pyrimidin-2-yl)piperazin- 1 -yl)butan- 1 -amine (5)
10.0 g of 8-(pyrimidin-2-yl)-5,8-diazaspiro[4,5]decan-5-ium bromide (11) (0.0334 moles), obtained according to US 4423049, is suspended in xylene (150 mL). 21.78 g of caesium carbonate (0.0668 moles) is then added. The resulting mixture is heated to 130°C and left under stirring for 60 minutes. 12.7 g of di-tert-butyl iminodicarboxylate (0.0584 moles) is then added and left under stirring until the reaction is complete. The mixture is cooled to about 80°C and filtered under vacuum, and the solid filtrate is washed with xylene (100 mL). 50 mL of 37% HC1 is added to the organic phase, and the resulting mixture is left under stirring for 10 min. The phases are then separated, and the organic phase is washed with a mixture of 50 mL of water and 5 mL of 37% HC1. 130 mL of dichloromethane is added to the aqueous acid phase and basified with 30% NaOH until pH = 13 is reached. The resulting mixture is left under stirring for 10 min., and the phases are separated. The aqueous phase is re-extracted with 200 mL of dichloromethane, and the combined organic phases are washed with 300 mL of water and 50 mL of brine, dried on sodium sulphate, filtered, and finally concentrated under vacuum to give 7.8 g of 4-(4-(pyrimidin-2-yl)piperazin-l-yl)butan-l-amine (5) (orange oil; yield 99%).
1H NMR (400 MHz, chloroform-d) d 8,17 (d, J = 4,7 Hz, 2H), 6,34 (t, J = 4,7 Hz, 1H), 3,76 – 3,63 (m, 4H), 2,60 (t, J = 6,9 Hz, 2H), 2,46 – 2,32 (m, 4H), 2,32 – 2,21 (m, 2H), 1,53 – 1,24 (m, 6H).
13C NMR (101 MHz, chloroform-d) d 161,55, 157,55, 109,65, 58,48, 53,02, 43,57, 42,01, 31,63, 24,18.
References
- ^ Jump up to:a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am “EXXUA (gepirone) extended-release tablets, for oral use” (PDF). Mission Pharmacal Company. U.S. Food and Drug Administration. 2023. Archived from the original (PDF) on 28 September 2023. Retrieved 28 September 2023.
- ^ Jump up to:a b c Kishi T, Meltzer HY, Matsuda Y, Iwata N (August 2014). “Azapirone 5-HT1A receptor partial agonist treatment for major depressive disorder: systematic review and meta-analysis” (PDF). Psychological Medicine. 44 (11): 2255–2269. doi:10.1017/S0033291713002857. PMID 24262766. S2CID 20830020. Archived from the original (PDF) on 18 February 2019.
- ^ Jump up to:a b Halbreich U, Montgomery SA (1 November 2008). Pharmacotherapy for Mood, Anxiety, and Cognitive Disorders. American Psychiatric Pub. pp. 375–. ISBN 978-1-58562-821-6.
- ^ Jump up to:a b c d e “Gepirone – Fabre-Kramer Pharmaceuticals”. AdisInsight. Springer Nature Switzerland AG. Archived from the original on 11 April 2023. Retrieved 28 September 2023.
- ^ Jump up to:a b c d e Becker Z (28 September 2023). “Decades long regulatory odyssey ends with FDA nod for Fabre-Kramer’s depression med Exxua”. Fierce Pharma.
- ^ Firth S (30 November 2015). “Controversial Antidepressant Comes Up for FDA OK — Again”. MedPage Today.
- ^ Kirsch I (2014). “Antidepressants and the Placebo Effect”. Zeitschrift für Psychologie. 222 (3): 128–134. doi:10.1027/2151-2604/a000176. PMC 4172306. PMID 25279271.
- ^ “FDA Rules Favorably On Efficacy Of Travivo (Gepirone ER) For Treatment Of Major Depressive Disorder”. Fabre-Kramer Pharmaceuticals, Inc. Cision PR Newswire. 17 March 2016.
- ^ “Gepirone”. Drugs and Lactation Database. National Institute of Child Health and Human Development. 2006. PMID 37856644. Retrieved 11 December 2023.
- ^ Schatzberg AF, Nemeroff CB (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. American Psychiatric Pub. pp. 494–. ISBN 978-1-58562-309-9.
- ^ Kaur Gill A, Bansal Y, Bhandari R, Kaur S, Kaur J, Singh R, et al. (July 2019). “Gepirone hydrochloride: a novel antidepressant with 5-HT1A agonistic properties”. Drugs of Today. 55 (7): 423–437. doi:10.1358/dot.2019.55.7.2958474. PMID 31347611. S2CID 198911377.
- ^ “Gepirone ER”. Adis Insight. Archived from the original on 6 August 2016. Retrieved 13 January 2016.
- ^ “FDA Rules Favorably On Efficacy Of Travivo (Gepirone ER) For Treatment Of Major Depressive Disorder” (Press release). 17 March 2016. Archived from the original on 24 September 2017. Retrieved 23 January 2018.
- ^ Jump up to:a b Fabre LF, Brown CS, Smith LC, Derogatis LR (May 2011). “Gepirone-ER treatment of hypoactive sexual desire disorder (HSDD) associated with depression in women”. The Journal of Sexual Medicine. 8 (5): 1411–1419. doi:10.1111/j.1743-6109.2011.02216.x. PMID 21324094.
- ^ Jump up to:a b Fabre LF, Clayton AH, Smith LC, Goldstein I, Derogatis LR (March 2012). “The effect of gepirone-ER in the treatment of sexual dysfunction in depressed men”. The Journal of Sexual Medicine. 9 (3): 821–829. doi:10.1111/j.1743-6109.2011.02624.x. PMID 22240272.
- Robinson DS, Sitsen JM, Gibertini M: A review of the efficacy and tolerability of immediate-release and extended-release formulations of gepirone. Clin Ther. 2003 Jun;25(6):1618-33. doi: 10.1016/s0149-2918(03)80159-5. [Article]
- Jenkins SW, Robinson DS, Fabre LF Jr, Andary JJ, Messina ME, Reich LA: Gepirone in the treatment of major depression. J Clin Psychopharmacol. 1990 Jun;10(3 Suppl):77S-85S. doi: 10.1097/00004714-199006001-00014. [Article]
- Yocca FD: Neurochemistry and neurophysiology of buspirone and gepirone: interactions at presynaptic and postsynaptic 5-HT1A receptors. J Clin Psychopharmacol. 1990 Jun;10(3 Suppl):6S-12S. [Article]
- FDA Approved Drug Products: EXXUA (gepirone) extended-release tablets, for oral use [Link]
- Gepirone US Patent Application Publication [Link]
- Fabre-Kramer Pharmaceuticals Announces FDA Approval of EXXUA™, the First and Only Oral Selective 5HT1a Receptor Agonist for the Treatment of Major Depressive Disorder in Adults [Link]
| Clinical data | |
|---|---|
| Trade names | Exxua |
| Other names | BMY-13805; MJ-13805; ORG-13011 |
| Routes of administration | By mouth[1] |
| ATC code | N06AX19 (WHO) |
| Legal status | |
| Legal status | US: ℞-only[1] |
| Pharmacokinetic data | |
| Bioavailability | 14–17%[1] |
| Protein binding | 72%[1] |
| Metabolism | CYP3A4[1] |
| Metabolites | 3′-OH-gepirone; 1-(2-Pyrimidinyl)piperazine[1] |
| Elimination half-life | IRTooltip Instant release: 2–3 hours ERTooltip Modified-release dosage: 5 hours[1] |
| Excretion | Urine: 81%[1] Feces: 13%[1] |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 83928-76-1 83928-66-9 |
| PubChem CID | 55191 |
| DrugBank | DB12184DBSALT002148 |
| ChemSpider | 49836 49835 |
| UNII | JW5Y7B8Z1880C9L8EP6V |
| KEGG | D04314 |
| ChEBI | CHEBI:135990 |
| ChEMBL | ChEMBL284092 ChEMBL1204187 |
| CompTox Dashboard (EPA) | DTXSID90232813 |
| Chemical and physical data | |
| Formula | C19H29N5O2 |
| Molar mass | 359.474 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
////////Gepirone, FDA 2023, APPROVALS 2023, Exxua, BMY 13805, MJ 13805, ORG 13011, BMY-13805, MJ-13805, ORG-13011, Gepirona, JW5Y7B8Z18














