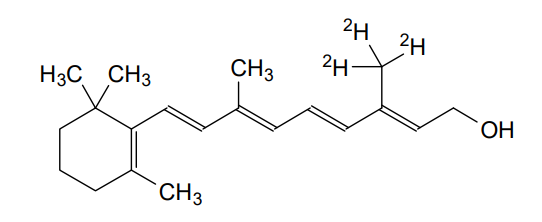
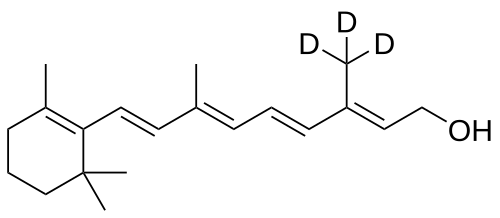
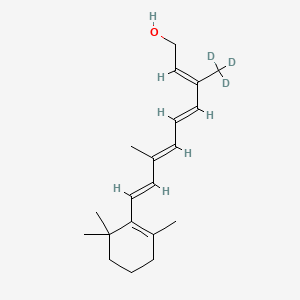
Gildeuretinol
CAS118139-35-8
MF C20H272H3O, MW 289.5 g/mol
(2E,4E,6E,8E)-3-(2H3)methyl-7-methyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol; (20,20,20-2H3)retinol
(2E,4E,6E,8E)-7-methyl-3-(trideuteriomethyl)-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraen-1-ol
vitamin A analogue, Orphan Drug, Stargardt disease, breakthrough therapy, Pediatric Rare Disease designations, ALK-001, KL-49, ALK 001, KL 49
- OriginatorColumbia University
- DeveloperAlkeus Pharmaceuticals
- ClassEye disorder therapies; Retinoids; Vitamins
- Mechanism of ActionDimerisation inhibitors; Vitamin A replacements
- Orphan Drug StatusYes – Stargardt disease
- Phase II/IIIDry age-related macular degeneration
- Phase IIStargardt disease
- No development reportedRetinal dystrophies
- 08 Sep 2025Gildeuretinol – Alkeus Pharmaceuticals receives Orphan Drug status for Stargardt disease in European Union
- 09 Jan 2025Alkeus Pharmaceuticals announces intention to submit an NDA to US FDA for Stargardt disease in 2025
- 09 Jan 2025Efficacy and adverse event data from phase II trial for Stargardt disease released by Alkeus Pharmaceuticals
Gildeuretinol is an investigational new drug being developed by Alkeus Pharmaceuticals, Inc. for the treatment of retinal diseases, particularly Stargardt disease and geographic atrophy secondary to age-related macular degeneration (AMD). Stargardt disease is caused by a defect in the ABCA4 gene that clears toxic byproducts resulting from the dimerization of vitamin A. Gildeuretinol is new molecular entity designed to reduce the dimerization of vitamin A in the eye without affecting the visual cycle.[1]
Gildeuretinol has received breakthrough therapy, orphan drug and Pediatric Rare Disease designations from the U.S. Food and Drug Administration.[2]



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Zaydon YA, Tsang SH (July 2024). “The ABCs of Stargardt disease: the latest advances in precision medicine”. Cell & Bioscience. 14 (1) 98. doi:10.1186/s13578-024-01272-y. PMC 11282698. PMID 39060921.
- Fitch J (22 November 2024). “Gildeuretinol for Stargardt disease receives Rare Pediatric Disease, Fast Track Designations”. Contemporary Pediatrics.
| Clinical data | |
|---|---|
| Other names | ALK-001, KL-49 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 118139-35-8 |
| PubChem CID | 169490774 |
| UNII | PSZ7W5NR24 |
| KEGG | D12713 |
| ChEMBL | ChEMBL5314606 |
| Chemical and physical data | |
| Formula | C20H30D3O |
| Molar mass | 292.500 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
/////////Gildeuretinol, vitamin A analogue, Orphan Drug, Stargardt disease, breakthrough therapy, Pediatric Rare Disease designations, ALK-001, KL-49, ALK 001, KL 49, PSZ7W5NR24














