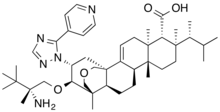
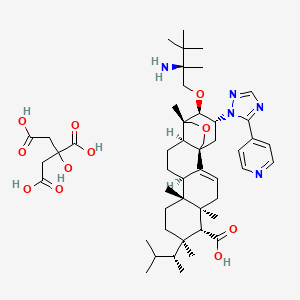
 Ibrexafungerp citrate
Ibrexafungerp citrate
|
アイブレキサフンジェルプクエン酸塩; |
| Formula |
C44H67N5O4. C6H8O7 |
|---|---|
| cas |
1965291-08-0
free 1207753-03-4 |
| Mol weight |
922.1574 |
- WHO 10597
- Originator Merck & Co; SCYNEXIS
- Class Antifungals; Glycosides; Triterpenes
- Mechanism of ActionBeta-1,3-D glucan synthetase inhibitors
- Orphan Drug StatusYes – Invasive bronchopulmonary aspergillosis; Candidiasis
- RegisteredVulvovaginal candidiasis
- Phase IIICandidiasis
- Phase IIInvasive bronchopulmonary aspergillosis
- Phase IUnspecified
- PreclinicalPneumocystis pneumonia
- 01 Jun 2021Registered for Vulvovaginal candidiasis (In adolescents, In children, In the elderly, In adults) in USA (PO)
- 01 May 2021Ibrexafungerp – SCYNEXIS receives Qualified Infectious Disease Product status for Vulvovaginal candidiasis (Recurrent, Prevention) in USA
- 30 Apr 2021Efficacy data from phase III VANISH-303 and VANISH-306 trials in Vulvovaginal Candidiasis presented at the 2021 American College of Obstetricians and Gynecologists Annual Meeting (ACOG-2021)
Medical uses
Ibrexafungerp is indicated for the treatment of adult and postmenarchal pediatric females with vulvovaginal candidiasis (VVC).[1][2] Syn https://www.sciencedirect.com/science/article/abs/pii/S0960894X20307721 SYN
SYN
Bioorg. Med. Chem. Lett. 2021, 32, 127661. PATENT
WO 2010019204
SYN
https://doi.org/10.1021/acs.jmedchem.3c00501
Ibrexafungerp (Brexafemme). Ibrexafungerp (1), formerly SCY-078 or MK-3118 and developed by Scynexis Inc., is a first-in-class triterpenoid antifungal that inhibits the biosynthesis of β-(1,3) D-glucan in the fungal cell wall. This mechanism of action provides an opportunity for the treatment of fungal infections that are azole- or echinocandrin-resistant strains. In June 2021, ibrexafungerp received its first approval by the United States Food and Drug Administration (USFDA) for the treatment of vulvovaginal candidiasis in adult and postmenarchal pediatric females. 24,25 Ibrexafungerp is a semisynthetic derivative of the natural product enfumafungin that incorporates a pyridine triazole moiety on the core phenanthropyran ring system as well as a pendant 2-amino-2,3,3trimethyl-butyl ether. The drug demonstrates potent, broad spectrum activity against Candida sp. and is orally bioavailable. As shown in Scheme 1, the synthesis of ibrexafungerp started with the natural product enfumafungin (1.1). The lactol of enfumafungin was reduced using triethylsilane and trifluoroacetic acid to give pyran 1.2. Treatmentwith H2SO4 in methanol resulted in cleavage of the glucose moiety to generate 1.3 in 87% yield over 2 steps. Carboxylic acid 1.3 was converted to the corresponding benzyl ester upon treatment with benzyl bromide to give compound 1.4in an89%yield. Reaction of 1.4 with (R) N-sulfonyl aziridine 1.5 (prepared as shown in Scheme 2) in the presence of potassium t-pentylate and the cation complexing agent 18-crown-6 provided ether 1.6 in 78% yield. Metal reduction with sodiumin liquid ammoniaconcurrently removed the N-sulfonyl benzyl groups to generate compound 1.7, which was converted to hydrazine intermediate 1.8 with anhydrous hydrazine and BF32·OEt 28-30 in 1,2-dichloroethane (DCE). Cyclocondensation of 1.8 with acyl amidine derivative 1.9 upon heating in acetic acid then provided ibrexafungerp (1) in 66% yield. Thepreparationof(R)-N-sulfonylaziridine1.5 isdescribedin Scheme 2. Condensation of 3,3-dimethylbutan-2-one (1.10)with (R)-p-toluenesulfinamide (1.11) gave an 84% yield of compound 1.12, which cyclized upon treatment with trimethylsulfoxonium chloride and n-butyllithium to give chiral toluenesulfinyl aziridine 1.13 in 64% yield. Oxidation of 1.13 with meta-chloroperoxybenzoic acid afforded the tosyl-pro tected (R)-alpha-disubstituted aziridine 1.5.. (24) Lee, A. Ibrexafungerp: First approval. Drugs 2021, 81, 1445− 1450. (25) Jallow, S.; Govender, N. P. Ibrexafungerp: A first-in-class oral triterpenoid glucan synthase inhibitor. J. Fungi 2021, 7, 163. (26) Lamoth, F.; Alexander, B. D. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non aspergillus mold isolates. Antimicrob. Agents Chemother. 2015, 59, 4308−4311
(27) Scorneaux, B.; Angulo, D.; Borroto-Esoda, K.; Ghannoum, M.; Peel, M.; Wring, S. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob. Agents Chemother. 2017, 61, e01961-16. (28) Apgar, J. M.; Wilkening, R. R.; Parker, D. L.; Meng, D.; Wildonger, K. J.; Sperbeck, D.; Greenlee, M. L.; Balkovec, J. M.; Flattery, A. M.; Abruzzo, G. K.; Galgoci, A. M.; Giacobbe, R. A.; Gill, C. J.; Hsu, M.-J.; Liberator, P.; Misura, A. S.; Motyl, M.; Nielsen Kahn, J.; Powles, M.; Racine, F.; Dragovic, J.; Fan, W.; Kirwan, R.; Lee, S.; Liu, H.; Mamai, A.; Nelson, K.; Peel, M. Ibrexafungerp: an orally active β 1,3-glucan synthesis inhibitor. Bioorg. Med. Chem. Lett. 2021, 32, 127661. (29) Greenlee, M. L.; Wilkening, R.; Apgar, J.; Sperbeck, D.; Wildonger, K. J.; Meng, D.; Parker, D. L.; Pacofsky, G. J.; Heasley, B. H.; Mamai, A.; Nelson, K. Antifungal Agents. WO 2010019204, 2010. (30) Greenlee, M. L.; Wilkening, R.; Apgar, J.; Wildonger, K. J.; Meng, D.; Parker, D. L. Antifungal Agents. WO 2010019203A1, 2010. (31) Imran, M.; Khan, S. A.; Alshammari, M. K.; Alqahtani, A. M.; Alanazi, T. A.; Kamal, M.; Jawaid, T.; Ghoneim, M. M.; Alshehri, S.; Shakeel, F. Discovery, development, inventions and patent review of fexinidazole: The first all-oral therapy for human African trypanoso miasis. Pharmaceuticals 2022, 15, 128.


. SYN European Journal of Medicinal Chemistry 245 (2023) 114898 The gram-scale synthesis of this drug is demonstrated in Scheme 3 [50]. Starting with triterpene glycoside enfumafungin 14, a reduction of the bridging hemiacetal with triethylsilane provided the intermediate 15, followed by hydrolysis, etherification and benzyl protection, gave compound 16 in 76% yield over 2 steps. Subsequent ring-opening alkylation reaction of 16 with tosyl protected aziridine 17 gave com pound 18, which then underwent Borch reduction to provide the in termediate 19. Treatment of 19 with biaryl 20 in the presence of boron trifluoride diethyl etherate gave rise to the substitution product ibrexafungerp. In this synthetic method, the pyridine-triazolium biaryl and chiral benzene sulfonamide were elegantly introduced into the triterpene enfumafungin through ring-opening and substitution reactions to give the triterpene derivative. These elegant and practical synthetic methods could be employed as the versatile tools for the synthesis of other drug molecules. [50] J.M. Apgar, R.R. Wilkening, D.L. Parker, J.D. Meng, K.J. Wildonger, D. Sperbeck, M.L. Greenlee, J.M. Balkovec, A.M. Flattery, G.K. Abruzzo, A.M. Galgoci, R. A. Giacobbe, C.J. Gill, M.J. Hsu, P. Liberator, A.S. Misura, M. Motyl, J.N. Kahn, M. Powles, F. Racine, J. Dragovic, W. Fan, R. Kirwan, S. Lee, H. Liu, A. Mamai, K. Nelson, M. Peel, Ibrexafungerp: an orally active β-1, 3-glucan synthesis inhibitor, Bioorg, Med. Chem. Lett. 32 (2021), 127661.

Abstract
We previously reported medicinal chemistry efforts that identified MK-5204, an orally efficacious β-1,3-glucan synthesis inhibitor derived from the natural product enfumafungin. Further extensive optimization of the C2 triazole substituent identified 4-pyridyl as the preferred replacement for the carboxamide of MK-5204, leading to improvements in antifungal activity in the presence of serum, and increased oral exposure. Reoptimizing the aminoether at C3 in the presence of this newly discovered C2 substituent, confirmed that the (R) t-butyl, methyl aminoether of MK-5204 provided the best balance of these two key parameters, culminating in the discovery of ibrexafungerp, which is currently in phase III clinical trials. Ibrexafungerp displayed significantly improved oral efficacy in murine infection models, making it a superior candidate for clinical development as an oral treatment for Candida and Aspergillus infections.
References
- ^ Jump up to:a b c d e f g https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214900s000lbl.pdf
- ^ Jump up to:a b c “Scynexis Announces FDA Approval of Brexafemme (ibrexafungerp tablets) as the First and Only Oral Non-Azole Treatment for Vaginal Yeast Infections”. Scynexis, Inc. (Press release). 2 June 2021. Retrieved 2 June 2021.
Further reading
- Azie N, Angulo D, Dehn B, Sobel JD (September 2020). “Oral Ibrexafungerp: an investigational agent for the treatment of vulvovaginal candidiasis”. Expert Opin Investig Drugs. 29 (9): 893–900. doi:10.1080/13543784.2020.1791820. PMID 32746636.
- Davis MR, Donnelley MA, Thompson GR (July 2020). “Ibrexafungerp: A novel oral glucan synthase inhibitor”. Med Mycol. 58 (5): 579–592. doi:10.1093/mmy/myz083. PMID 31342066.
- Petraitis V, Petraitiene R, Katragkou A, Maung BB, Naing E, Kavaliauskas P, et al. (May 2020). “Combination Therapy with Ibrexafungerp (Formerly SCY-078), a First-in-Class Triterpenoid Inhibitor of (1→3)-β-d-Glucan Synthesis, and Isavuconazole for Treatment of Experimental Invasive Pulmonary Aspergillosis”. Antimicrob Agents Chemother. 64 (6). doi:10.1128/AAC.02429-19. PMC 7269506. PMID 32179521.
External links
- “Ibrexafungerp”. Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03734991 for “Efficacy and Safety of Oral Ibrexafungerp (SCY-078) vs. Placebo in Subjects With Acute Vulvovaginal Candidiasis (VANISH 303)” at ClinicalTrials.gov
- Clinical trial number NCT03987620 for “Efficacy and Safety of Oral Ibrexafungerp (SCY-078) vs. Placebo in Subjects With Acute Vulvovaginal Candidiasis (Vanish 306)” at ClinicalTrials.gov
- Wring SA, Randolph R, Park S, Abruzzo G, Chen Q, Flattery A, Garrett G, Peel M, Outcalt R, Powell K, Trucksis M, Angulo D, Borroto-Esoda K: Preclinical Pharmacokinetics and Pharmacodynamic Target of SCY-078, a First-in-Class Orally Active Antifungal Glucan Synthesis Inhibitor, in Murine Models of Disseminated Candidiasis. Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: AAC.02068-16. doi: 10.1128/AAC.02068-16. Print 2017 Apr. [Article]
- Hector RF, Bierer DE: New beta-glucan inhibitors as antifungal drugs. Expert Opin Ther Pat. 2011 Oct;21(10):1597-610. doi: 10.1517/13543776.2011.603899. Epub 2011 Jul 25. [Article]
- Kuhnert E, Li Y, Lan N, Yue Q, Chen L, Cox RJ, An Z, Yokoyama K, Bills GF: Enfumafungin synthase represents a novel lineage of fungal triterpene cyclases. Environ Microbiol. 2018 Sep;20(9):3325-3342. doi: 10.1111/1462-2920.14333. Epub 2018 Sep 13. [Article]
- Larkin EL, Long L, Isham N, Borroto-Esoda K, Barat S, Angulo D, Wring S, Ghannoum M: A Novel 1,3-Beta-d-Glucan Inhibitor, Ibrexafungerp (Formerly SCY-078), Shows Potent Activity in the Lower pH Environment of Vulvovaginitis. Antimicrob Agents Chemother. 2019 Apr 25;63(5). pii: AAC.02611-18. doi: 10.1128/AAC.02611-18. Print 2019 May. [Article]
- Ha YS, Covert SF, Momany M: FsFKS1, the 1,3-beta-glucan synthase from the caspofungin-resistant fungus Fusarium solani. Eukaryot Cell. 2006 Jul;5(7):1036-42. doi: 10.1128/EC.00030-06. [Article]
- Perlin DS: Mechanisms of echinocandin antifungal drug resistance. Ann N Y Acad Sci. 2015 Sep;1354:1-11. doi: 10.1111/nyas.12831. Epub 2015 Jul 17. [Article]
- Wring S, Murphy G, Atiee G, Corr C, Hyman M, Willett M, Angulo D: Clinical Pharmacokinetics and Drug-Drug Interaction Potential for Coadministered SCY-078, an Oral Fungicidal Glucan Synthase Inhibitor, and Tacrolimus. Clin Pharmacol Drug Dev. 2019 Jan;8(1):60-69. doi: 10.1002/cpdd.588. Epub 2018 Jun 27. [Article]
- Ghannoum M, Arendrup MC, Chaturvedi VP, Lockhart SR, McCormick TS, Chaturvedi S, Berkow EL, Juneja D, Tarai B, Azie N, Angulo D, Walsh TJ: Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections. Antibiotics (Basel). 2020 Aug 25;9(9). pii: antibiotics9090539. doi: 10.3390/antibiotics9090539. [Article]
- FDA Approved Drug Products: Brexafemme (Ibrexafungerp) Oral Tablet [Link]
 |
|
| Clinical data | |
|---|---|
| Trade names | Brexafemme |
| Other names | SCY-078 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Antifungal |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL |
|
| Chemical and physical data | |
| Formula | C44H67N5O4 |
| Molar mass | 730.051 g·mol−1 |
| 3D model (JSmol) | |
|
|
|
|



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on ResearchgateRESEARCHGATE

Anthony Melvin Crasto Dr. | Facebook
join me on twitter Anthony Melvin Crasto Dr. | twitter +919321316780 call whatsaapp EMAIL. amcrasto@gmail.com














