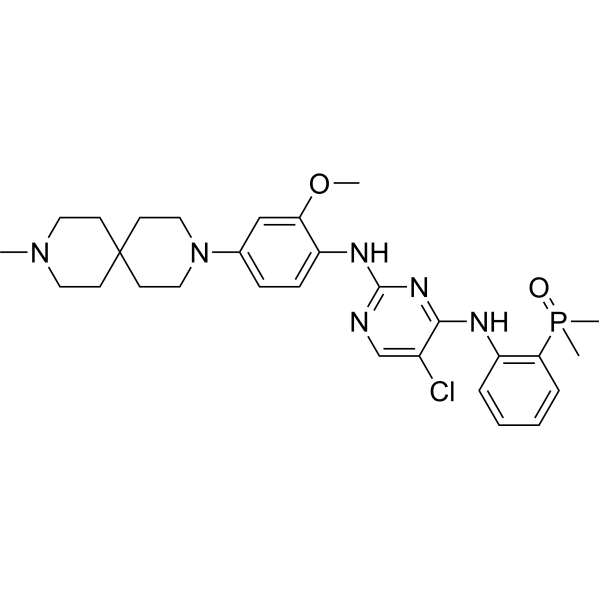
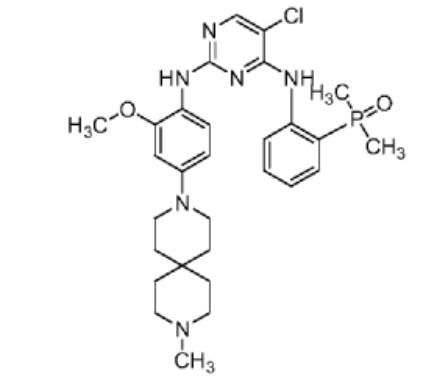
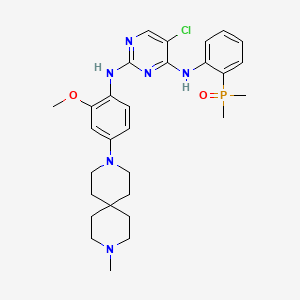
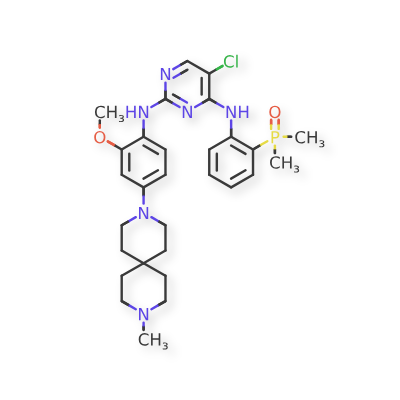
Iruplinalkib
CAS No. : 1854943-32-0
| Molecular Weight | 569.08 |
|---|---|
| Formula | C29H38ClN6O2P |
5-chloro-4-N-(2-dimethylphosphorylphenyl)-2-N-[2-methoxy-4-(9-methyl-3,9-diazaspiro[5.5]undecan-3-yl)phenyl]pyrimidine-2,4-diamine
Iruplinalkib (WX-0593) is an orally active and selective ALK/ROS1 inhibitor. Iruplinalkib can effectively inhibit tyrosine autophosphorylation of ALK and mutant ALK, EGFR, with the IC50 between 5.38 and 16.74 nM. Iruplinalkib is also a suppressive agent of the transporter MATE1, MATE2K, P-gp and BCRP. Iruplinalkib can be used in the study of non-small cell lung cancer.
Iruplinalkib is an orally available, small molecule inhibitor of the receptor tyrosine kinase (RTK) anaplastic lymphoma kinase (ALK), with potential antineoplastic activity. Upon oral administration, iruplinalkib binds to and inhibits ALK tyrosine kinase, ALK fusion proteins, ALK point mutation variants ALK L1196M, ALK C1156Y, and EGFR L858R/T790M. Inhibition of ALK leads to the disruption of ALK-mediated signaling and the inhibition of cell growth in ALK-expressing tumor cells. ALK belongs to the insulin receptor superfamily and plays an important role in nervous system development. ALK is not expressed in healthy adult human tissue but ALK dysregulation and gene rearrangements are associated with a series of tumors. Additionally, ALK mutations are associated with acquired resistance to small molecule tyrosine kinase inhibitors.
SYN
Bioorg. Chem. 2023, 140, No. 106807
Bioorg. Med. Chem. Lett. 2022, 66, No. 128730.
CN106928275, 2017.
EP 3165530 A1, 2017
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US196228122&_cid=P22-ME5G3V-88608-1
Example 9
(2-((5-Chloro-2-((2-methoxy-4-(9-methyl-3,9-diazaspiro[5.5]undecan-3-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)dimethyl phosphine oxide
This Example was prepared according to the process as described in Example 7, take the place of (2-((5-chloro-2-((2-methoxy-4-(2,6-diazaspiro[3.4]octan-6-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)dimethyl phosphine oxide was replaced with (2-((5-chloro-2-((2-methoxy-4-(3,9-diazaspiro[5.5]undecan-3-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)dimethyl phosphine oxide to give the title compound as yellow solid, yield 57%. 1H NMR (400 MHz, CD 3OD): δ, 8.28 (s, 1H), 8.12 (br. s., 1H), 7.81-7.68 (m, 3H), 7.65 (d, J=2.0 Hz, 1H), 7.56-7.49 (m, 1H), 7.37 (d, J=8.8 Hz, 1H), 4.02 (s, 3H), 3.74 (br. s., 4H), 3.47 (d, J=12.8 Hz, 2H), 3.24 (t, J=12.8 Hz, 2H), 2.93 (s, 3H), 2.42-1.97 (m, 5H), 1.93-1.75 (m, 9H). LCMS (ESI) (0-60AB): m/z: 569.2 [M+1].
PATENT
CN 110407877, 2019
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN276468167&_cid=P22-ME5FTE-83156-1
| Experimental Example 11 |
| (2-((5-chloro-2-((2-methoxy-4-(9-methyl-3,9-diaza-spiro[5.5]undec-3-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide (Compound I) |
| NMP (N-methylpyrrolidone, 102.6 g) was added to the reaction bottle (room temperature), and 20 g of compound (e) was added to the reaction bottle under stirring. 21.85 g of compound (f) was added to the reaction bottle, and 19.93 g (3 eq) of MeSO 3 H was added dropwise to the reaction flask (temperature was controlled at <40°C) 2 Bubble for 15-20 minutes. Heat the reaction to 85-90°C and react at 85-90°C for 12 hours before sampling and testing (HPLC). Sample testing (test method: dissolve 0.1 ml of the reaction solution in 2 ml of MeOH). Stop the reaction when compound (f) is <2.5%. Add NaOH solution (1M, 287 g) to the reaction solution to adjust the pH to 13. A large amount of solid precipitates and continue stirring for 2 hours. Filter the solid and wash the filter cake with water (40 g) until the filtrate is colorless. Collect the filter cake, filter and dry (55-60°C) to obtain an off-white crude product. Add methanol (268 g) to the reaction flask, then add the obtained solid, heat to 60°C to dissolve, add activated carbon (5.2 g) to the reaction flask, then stir at 60°C for 2.5 hours, cool to 30°C, filter, and concentrate the mother liquor under reduced pressure to obtain a gray-green solid. MeOH (155.5 g) was added to the reaction flask, followed by the solid obtained above. The mixture was heated under reflux until clear. Purified water (388 g) was added to the reaction flask and the temperature was naturally lowered to 15-20°C. A white solid precipitated. The solid was filtered and washed once with purified water (194 g). The filter cake was collected and dried to yield Compound I (32.5 g). 1 H NMR (400MHz, CD3OD): 8.36 (dd, J=8.0, 4.4Hz, 1H), 8.03 (s, 1H), 7.69 (d, J=8.8Hz, 1H), 7.65-7.55 (m, 1H), 7.51 (dd, J=8.0 8.0Hz,1H),7.32-7.20(m,1H),6.66(d,J=2.4Hz,1H),6.45(dd,J=8.8,2.4Hz,1H),3.85(s,3H) ,3.18-3.06(m,4H),2.54-2.38(m,4H),2.30(s,3H),1.85(d,J=13.6Hz,6H),1.74-1.54(m,8H). |
| Example 1: Preparation of Form A of Compound I: |
| 10 g of (2-((5-chloro-2-((2-methoxy-4-(9-methyl-3,9-diaza-spiro[5.5]undec-3-yl)phenyl)amino)pyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide (Compound I) was heated to reflux for complete dissolution with 45 ml of ethanol and 20 ml of purified water. The mixture was then cooled to 10-20° C., 50 ml of purified water was added, and the mixture was stirred at this temperature for 2-3 hours. The mixture was filtered and dried in vacuo at 50-60° C. to obtain 9.0 g of an off-white solid with a yield of 90%. X-ray powder diffraction analysis showed an XRPD pattern as shown in FIG1 . DSC-TGA analysis yielded pattern 2. |
Syn
https://doi.org/10.1021/acs.jmedchem.4c02079
J. Med. Chem. 2025, 68, 2147−2182
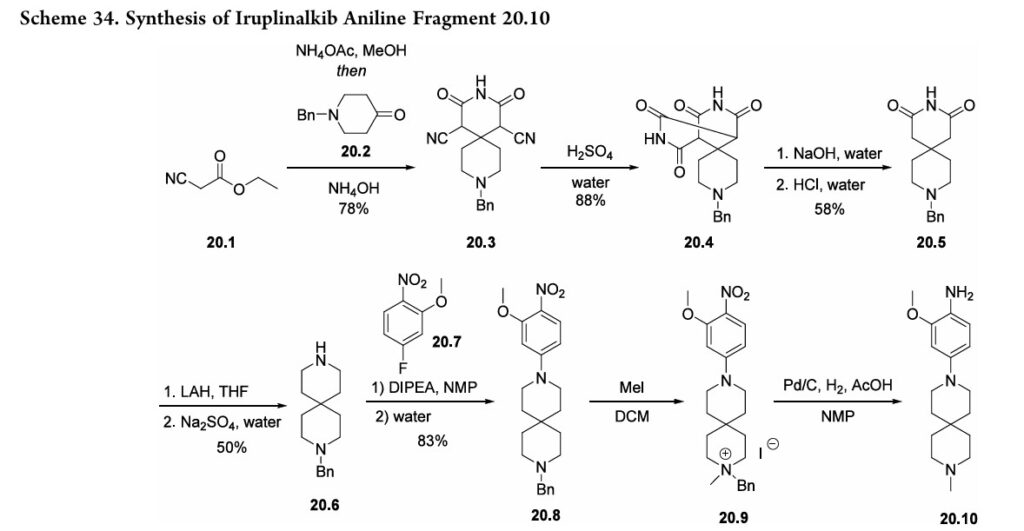
Iruplinalkib. The final NSCLC treatment approved in 2023 was iruplinalkib (20), developed by Qilu Pharmaceutical Co., Ltd. and approved for use in China in June 2023.156,157 Iruplinalkib is a highly selective oral anaplastic kinase (ALK) and ROS1TKI,andis, therefore, an oral treatment for ALK-positive (ALK+) or ROS1-positive (ROS1+) NSCLC.156,157 This treatment is specifically meant for use by patients with locally advanced or metastatic ALK-positive NSCLC whose disease has progressed after crizotinib therapy, often due to the develop ment of crizotinib resistance. Although early syntheses were published 158 further optimized routes were disclosed by Qilu Pharmaceutical Co., Ltd.(Scheme 34 and Scheme 35).First, ethyl 2-cyanoacetate 20.1) and cyclic ketone20.2 were combined to generate spirocyclic imide 20.3 (Scheme 34). The cyano substituents were then removed by treating 20.3 with sulfuric acid followed
by sodiumhydroxide to form 20.5. Subsequent amide reduction via lithium aluminum hydride treatment revealed secondary amine 20.6, which underwent a substitution reaction with fluorophenyl 20.7 to produce intermediate 20.8. Next, addition of methyl iodide generated a quaternary amine (20.9). The
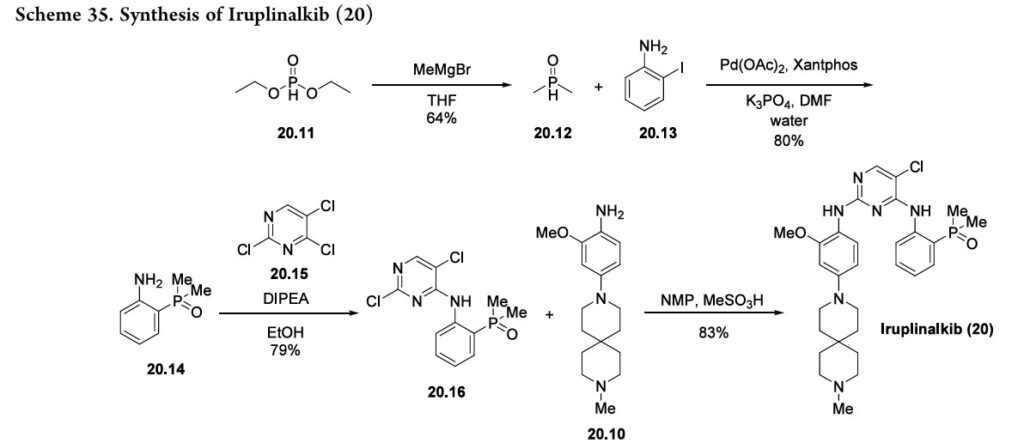
following hydrogenolysis step reduced the nitro group and removed the benzyl substituent using palladium on carbon to produce the final aniline fragment, 20.10. To generate the other fragment (20.16) to construct iruplinalkib, diethylphosphite (20.11) and methyl magnesium bromide were combinedtoproducedimethylphosphineoxide 20.12(Scheme35).Couplingthisproductwith2-iodoaniline
20.13andsubsequent selectiveSNArwithtrichloropyrimidine20.15 generated the resultingdichloropyrimidine20.16.Finally, a selective SNAr reaction yielded the desired product,
iruplinalkib(20).
(156) Yang, Y.; Zheng, Q.; Wang, X.; Zhao, S.; Huang, W.; Jia, L.; Ma,
C.; Liu, S.; Zhang, Y.; Xin, Q.; et al. Iruplinalkib (WX-0593), a novel
ALK/ROS1 inhibitor, overcomes crizotinib resistance in preclinical
modelsfornon-smallcell lung cancer. Invest. New Drugs 2023, 41,254−
266.
(157) Keam, S. J. Iruplinalkib: first approval. Drugs 2023, 83, 1717−
1721.
(158) Liu, X.; Zhang, L.; Wan, H.; Zhu, Z.; Jin, J.; Qin, Y.; Mao, W.;
Yan, K.; Fang, D.; Jiang, W.; et al. Discovery and preclinical evaluations
of WX-0593, a novel ALK inhibitor targeting crizotinib-resistant
mutations. Bioorg. Med. Chem. Lett. 2022, 66, No. 128730.
(159) Ding, Z.; Chen, S.; Liu, X.; Wan, H.; Zhang, L. Preparation,
intermediate and crystal form of spiroamine type arylphosphine oxide.
CN106928275, 2017.
(160) Ding, Z.; Zhang, M.; Chen, S.; Liu, X.; Zhu, Y.; Fan, C.;
Baoping, Z.; Chang, L.; Yang, Y.; Zheng, Q.; et al. Preparation,
intermediate and crystal form of spiroamine type arylphosphine oxide.
EP 3165530 A1, 2017.
(161) Lin, D.; Zhou, G.; Li, S.; Wang, X.; Zhang, Z.; Liu, Z.; Wang, X.
Polymorph of spirocycloaryl phosphorus oxide. CN 110407877, 2019.
(162) Gao, H.; Zhang, J.-Y.; Zhao, L.-J.; Guo, Y.-Y. Synthesis and
clinical application of small-molecule inhibitors and PROTACs of
anaplastic lymphoma kinase. Bioorg. Chem. 2023, 140, No. 106807.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
- [1]. Yang Y, et al. Iruplinalkib (WX 0593), a novel ALK/ROS1 inhibitor, overcomes crizotinib resistance in preclinical models for non-small cell lung cancer. Invest New Drugs. 2023 Apr;41(2):254-266. [Content Brief][2]. Shi Y, et al. Safety and activity of WX-0593 (Iruplinalkib) in patients with ALK- or ROS1-rearranged advanced non-small cell lung cancer: a phase 1 dose-escalation and dose-expansion trial. Signal Transduct Target Ther. 2022;7(1):25. [Content Brief]
/////////Iruplinalkib, china 2023, approvals 2023, Qilu Pharmaceutical, Z5F65W1YAZ, Qixinke, WX0593, WX 0593, FL006, FL 006, FL-006














