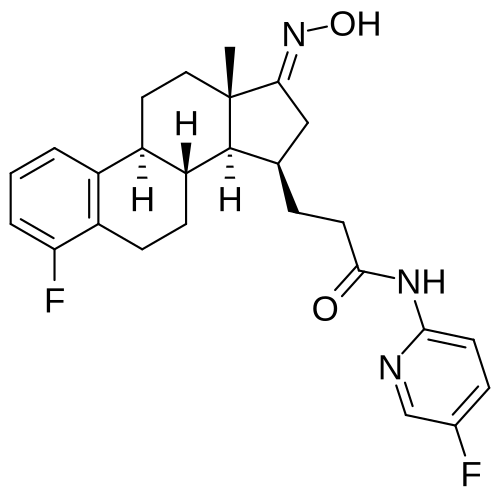
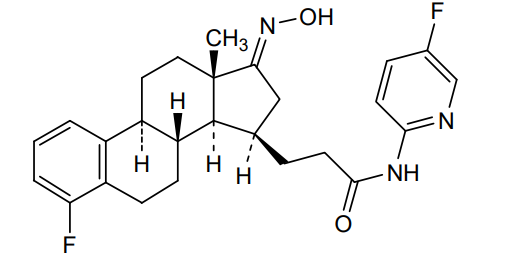
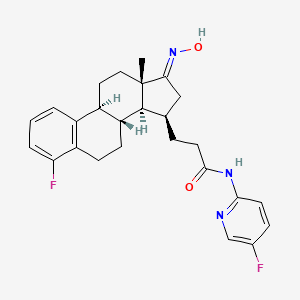
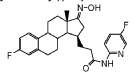
Linustedastat
CAS 2254299-48-2
MFC26H29F2N3O2 MW 453.5 g/mol
FOR-6219, OG-6219, FOR 6219, OG 6219, PP3PLL7GZY, Phase 2, Endometriosis
3-[(8R,9S,13S,14S,15R,17E)-4-fluoro-17-hydroxyimino-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-15-yl]-N-(5-fluoro-2-pyridinyl)propanamide
- (15beta,17E)-4-Fluoro-N-(5-fluoro-2-pyridinyl)-17-(hydroxyimino)estra-1,3,5(10)-triene-15-propanamide
- 3-[(17E)-4-fluoro-17-(hydroxyimino)estra-1,3,5(10)-trien-15beta-yl]-N-(5-fluoropyridin-2-yl)propanamide
- Estra-1,3,5(10)-triene-15-propanamide, 4-fluoro-N-(5-fluoro-2-pyridinyl)-17-(hydroxyimino)-, (15beta,17E)-
3-[(17E)-4-fluoro-17-(hidroxiimino)estra-1,3,5(10)-trien-15β-il]-N-(5-fluoropiridin-2-il)propanamida
inhibidor de la hidroxiesteroide 17-beta deshidrogenasa 1(HSD17B1)
- OriginatorHormos Medical; Solvay Pharmaceuticals B.V.; University of Turku
- DeveloperOrganon
- ClassSmall molecules
- Mechanism of ActionEstradiol dehydrogenase inhibitors
- Phase IIEndometriosis
- 02 Jul 2025Efficacy data from the phase II ELENA trial in Endometriosis released by Organon
- 28 May 2025Organon completes a phase-II clinical trials in Endometriosis (In adults) in Latvia, Sweden, Poland, Italy, France, Hungary, Germany, Czech Republic, Czech Republic, Bulgaria, Belgium, USA (PO) (NCT05560646)
- 28 Nov 2023No recent reports of development identified for phase-I development in Endometriosis(In volunteers) in United Kingdom (PO)
Linustedastat (developmental code names FOR-6219 and OG-6219) is a 17β-hydroxysteroid dehydrogenase 1 (17β-HSD1; HSD17B1) inhibitor which is under development for the treatment of endometriosis.[1][2][3][4][5] It is a steroidal compound derived from estrone and works by preventing the formation of the more potent estrogen estradiol from the minimally active precursor estrone.[1][2][5] This in turn results in antiestrogenic effects that may be useful in the treatment of estrogen-dependent conditions.[1][2][5] As of November 2023, the drug is in phase 2 clinical trials for endometriosis.[1][2] It is also under preclinical investigation for treatment of breast cancer and endometrial cancer.[5]
A Study to Investigate Efficacy and Safety of OG-6219 BID in 3 Dose Levels Compared With Placebo in Participants Aged 18 to 49 With Moderate to Severe Endometriosis-related Pain
CTID: NCT05560646
Phase: Phase 2
Status: Completed
Date: 2025-05-29
Pat
WO2018224736
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018224736&_cid=P21-MHFVBM-49409-1
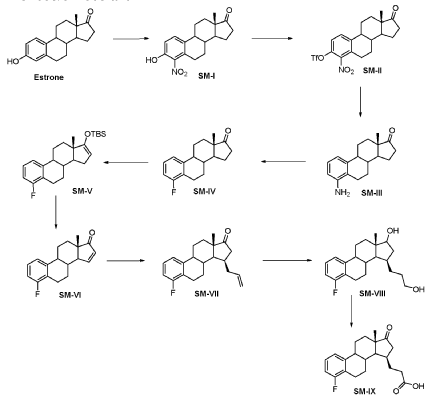
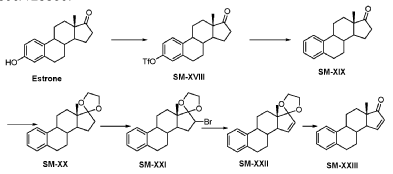
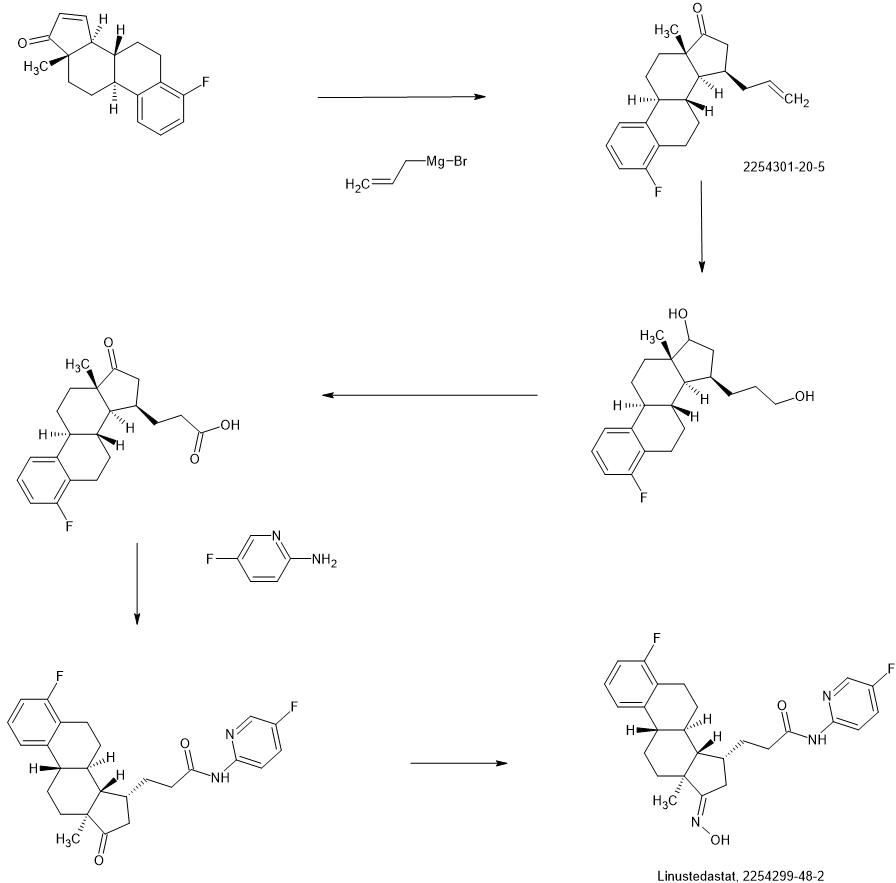
Compound 26
3-((13S,15R,E)-3-fluoro-17-(hydroxyimino)-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-15-yl)-N-(5-fluoropyridin-2-yl)propanamide
Example 26 was prepared in 94% yield from the compound 25 by the same method as with Example 2 in three hours reaction time.
1H NMR (200 MHz, DMSO-d6): 1.03 (s, 3 H), 1.12 – 2.48 (m, 15 H), 2.57 – 2.78 (m, 1 H), 2.80 – 2.95 (m, 2 H), 6.79 – 7.01 (m, 2 H), 7.18 – 7.38 (m, 1 H), 7.72 (td, 1 H), 8.15 (dd, 1 H), 8.31 (d, 1 H), 10.18 (s, 1 H), 10.64 (s, 1 H). MS m/z (TOF ES+): 454 (M+1).
SYNTHESIS



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | FOR-6219; OG-6219; 3-[(17E)-4-Fluoro-17-(hydroxyimino)estra-1,3,5(10)-trien-15β-yl]-N-(5-fluoropyridin-2-yl)propanamide |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2254299-48-2 |
| PubChem CID | 171390018 |
| UNII | PP3PLL7GZY |
| KEGG | D13078 |
| Chemical and physical data | |
| Formula | C26H29F2N3O2 |
| Molar mass | 453.534 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- “FOR 6219”. AdisInsight. 28 November 2023. Retrieved 15 August 2024.
- “Delving into the Latest Updates on Linustedastat with Synapse”. Synapse. 3 August 2024. Retrieved 15 August 2024.
- Barra F, Romano A, Grandi G, Facchinetti F, Ferrero S (June 2019). “Future directions in endometriosis treatment: discovery and development of novel inhibitors of estrogen biosynthesis”. Expert Opin Investig Drugs. 28 (6): 501–504. doi:10.1080/13543784.2019.1618269. hdl:11380/1201688. PMID 31072144.
- Perrone U, Evangelisti G, Laganà AS, Bogliolo S, Ceccaroni M, Izzotti A, Gustavino C, Ferrero S, Barra F (December 2023). “A review of phase II and III drugs for the treatment and management of endometriosis”. Expert Opin Emerg Drugs. 28 (4): 333–351. doi:10.1080/14728214.2023.2296080. PMID 38099328.
- Rižner TL, Romano A (2023). “Targeting the formation of estrogens for treatment of hormone dependent diseases-current status”. Front Pharmacol. 14 1155558. doi:10.3389/fphar.2023.1155558. PMC 10175629. PMID 37188267.
Several compounds with inhibitory action on the enzyme HSD17B1 have been developed and one steroidal compound, a competitive HSD17B1 inhibitor (OG-6219) recently entered the clinical phase for endometriosis […] and it is in the preclinical phase for endometrial and breast cancer (Husen et al., 2006a; Husen et al., 2006b; Konings et al., 2018b; Jarvensivu et al., 2018; Xanthoulea et al., 2021). […] Only the C15 estrone derivative developed by Organon Finland, former Forendo pharma (compound FOR-6219/OR-6219) reached the clinical phase for endometriosis with three clinical trials registered in the database Clinical Trails (Table 2). Phase 1 and 1b trials NCT04686669 and NCT03709420 determined the bio-availability of the compound administered orally as gelatine capsule in 12 subjects (NCT04686669) and then the safety, tolerability, food interactions, the pharmacokinetics and pharmacodynamics of escalating doses of the drug in 87 subjects (NCT03709420). The phase 2 randomized, double-blind, Elena study (NCT05560646) is currently recruiting patients and aims at evaluating the efficacy and safety of OG-6219 in women with moderate to severe endometriosis […]
External links
//////////Linustedastat, FOR-6219, OG-6219, FOR 6219, OG 6219, PP3PLL7GZY, Phase 2, Endometriosis














