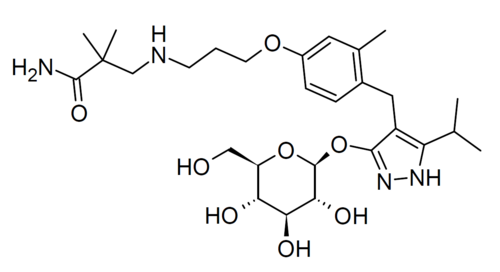
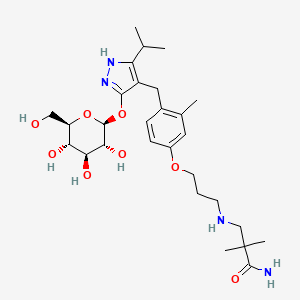
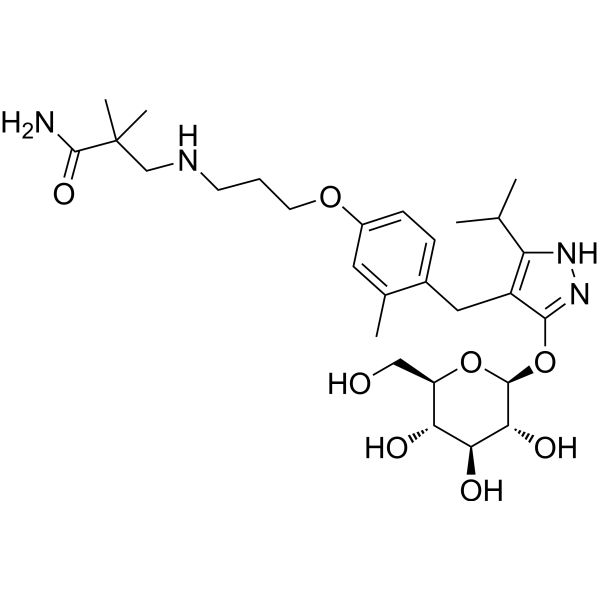
Mizagliflozin
- CAS 666843-10-3
- 1X96A704XV
- DSP-3235
- KGA-3235
WeightAverage: 564.68
Monoisotopic: 564.315914393
Chemical FormulaC28H44N4O8
- Dsp-3235 free base
- GSK-1614235 free base
- Kga-3235 free base
2,2-dimethyl-3-[3-[3-methyl-4-[[5-propan-2-yl-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1H-pyrazol-4-yl]methyl]phenoxy]propylamino]propanamide
- 3-((3-(4-((3-(beta-D-Glucopyranosyloxy)-5-(propan-2-yl)-1H-pyrazol-4-yl)methyl)-3-methylphenoxy)propyl)amino)-2,2-dimethylpropanamide
- Propanamide, 3-((3-(4-((3-(beta-D-glucopyranosyloxy)-5-(1-methylethyl)-1H-pyrazol-4-yl)methyl)-3-methylphenoxy)propyl)amino)-2,2-dimethyl-
Mizagliflozin is an SGLT1 inhibitor developed as a potential treatment for chronic constipation.[1][2] It progressed as far as Phase II trials in humans but was not approved for medical use, however it has since been investigated for other applications.[3][4]
MIZAGLIFLOZIN is a small molecule drug with a maximum clinical trial phase of II and has 1 investigational indication.
Mizagliflozin is under investigation in clinical trial NCT05721729 (Effect of Mizagliflozin Repeat Dosing on Adverse Events and Postprandial Glucose Excursions).
an SGLT1 inhibitor; structure in first source
- OriginatorKissei Pharmaceutical
- DeveloperKissei Pharmaceutical; Vogenx
- ClassAmides; Antihypoglycaemics; Laxatives; Pyrazoles; Small molecules
- Mechanism of ActionSodium-glucose transporter 1 inhibitors
- Phase IIHypoglycaemia
- Phase IGastroparesis
- PreclinicalUnspecified
- DiscontinuedConstipation
- 18 Jun 2025Phase-I clinical trials in Gastroparesis in USA (PO) (Vogenx pipeline, June 2025)
- 18 Jun 2025Preclinical trials in Undisclosed rare disease in USA (PO) (Vogenx pipeline, June 2025)
- 01 Oct 2019Chemical structure information added
LIT
- Pyrazole derivatives, medicinal composition containing the same, medicinal use thereof, and intermediate for production thereofPublication Number: US-7635684-B2Priority Date: 2002-08-23Grant Date: 2009-12-22
- Pyrazole derivatives, medicinal composition containing the same, medicinal use thereof, and intermediate for production thereofPublication Number: US-8324176-B2Priority Date: 2002-08-23Grant Date: 2012-12-04
- Monosebacate of pyrazole derivativePublication Number: US-2010279962-A1Priority Date: 2007-12-27
- Monosebacate of pyrazole derivativePublication Number: US-8399418-B2Priority Date: 2007-12-27Grant Date: 2013-03-19
- Monosebacate of pyrazole derivativePublication Number: WO-2009084531-A1Priority Date: 2007-12-27
- Pyrazole derivatives, medicinal composition containing the same, medicinal use thereof, and intermediate for production thereofPublication Number: US-2005272669-A1Priority Date: 2002-08-23
- Pyrazole derivatives, medicinal composition containing the same, medicinal use thereof, and intermediate for production thereofPublication Number: US-2009203633-A1Priority Date: 2002-08-23
- Hemifumarate of a pyrazole derivativePublication Number: WO-2009128421-A1Priority Date: 2008-04-16
- Monosebacate of pyrazole derivativePublication Number: AU-2008344436-B2Priority Date: 2007-12-27Grant Date: 2013-08-29
- Monosebacate of pyrazole derivativePublication Number: EP-2228378-A1Priority Date: 2007-12-27
- Monosebacate salt of pyrazole derivativePublication Number: JP-5144683-B2Priority Date: 2007-12-27Grant Date: 2013-02-13
- Monosebacate salt of pyrazole derivativePublication Number: JP-WO2009084531-A1Priority Date: 2007-12-27
- Hemifumarate of a pyrazole derivativePublication Number: EP-2275430-B1Priority Date: 2008-04-16Grant Date: 2012-05-16
- 1/2 fumarate salt of pyrazole derivativePublication Number: JP-5467040-B2Priority Date: 2008-04-16Grant Date: 2014-04-09
- 1/2 fumarate salt of pyrazole derivativePublication Number: JP-WO2009128421-A1Priority Date: 2008-04-16
- Hemifumarate of a pyrazole derivativePublication Number: US-2011034679-A1Priority Date: 2008-04-16
- Hemifumarate of a pyrazole derivativePublication Number: US-8354382-B2Priority Date: 2008-04-16Grant Date: 2013-01-15



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Legal status | |
|---|---|
| Legal status | Investigational |
| Identifiers | |
| IUPAC name | |
| CAS Number | 666843-10-3 |
| PubChem CID | 10460535 |
| ChemSpider | 8635948 |
| UNII | 1X96A704XV |
| ChEMBL | ChEMBL5314923 |
| Chemical and physical data | |
| Formula | C28H44N4O8 |
| Molar mass | 564.680 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- Inoue T, Takemura M, Fushimi N, Fujimori Y, Onozato T, Kurooka T, et al. (July 2017). “Mizagliflozin, a novel selective SGLT1 inhibitor, exhibits potential in the amelioration of chronic constipation”. European Journal of Pharmacology. 806: 25–31. doi:10.1016/j.ejphar.2017.04.010. PMID 28410751.
- Fukudo S, Endo Y, Hongo M, Nakajima A, Abe T, Kobayashi H, et al. (September 2018). “Safety and efficacy of the sodium-glucose cotransporter 1 inhibitor mizagliflozin for functional constipation: a randomised, placebo-controlled, double-blind phase 2 trial”. The Lancet. Gastroenterology & Hepatology. 3 (9): 603–613. doi:10.1016/S2468-1253(18)30165-1. PMID 30056028.
- Ishida N, Saito M, Sato S, Tezuka Y, Sanbe A, Taira E, et al. (October 2021). “Mizagliflozin, a selective SGLT1 inhibitor, improves vascular cognitive impairment in a mouse model of small vessel disease”. Pharmacology Research & Perspectives. 9 (5): e00869. doi:10.1002/prp2.869. PMC 8480397. PMID 34586752.
- Tsunokake S, Iwabuchi E, Miki Y, Kanai A, Onodera Y, Sasano H, et al. (October 2023). “SGLT1 as an adverse prognostic factor in invasive ductal carcinoma of the breast”. Breast Cancer Research and Treatment. 201 (3): 499–513. doi:10.1007/s10549-023-07024-9. PMID 37439959.
- [1]. Inoue T, et al. Mizagliflozin, a novel selective SGLT1 inhibitor, exhibits potential in the amelioration of chronic constipation. Eur J Pharmacol. 2017 Jul 5;806:25-31. [Content Brief][2]. Ohno H, et al. Absorption, disposition, metabolism and excretion of [14C]mizagliflozin, a novel selective SGLT1 inhibitor, in rats. Xenobiotica. 2019 Apr;49(4):463-473. [Content Brief]
/////////666843-10-3, 1X96A704XV, DSP 3235, KGA 3235, Mizagliflozin, Dsp-3235 free base, GSK-1614235 free base, Kga-3235 free base














