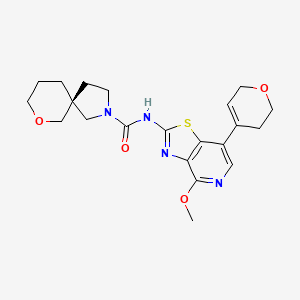
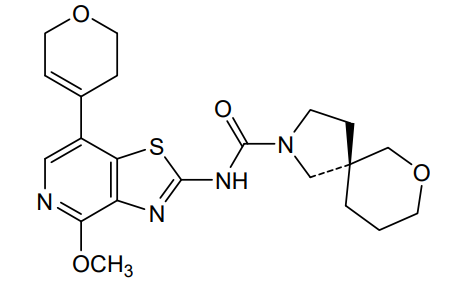
Muvadenant
CAS 2459881-03-7
MF C21H26N4O4S , 430.5 g/mol
(5S)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy[1,3]thiazolo[4,5-c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5] decane-2-carboxamide
(5S)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1,3]thiazolo[4,5-c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide
adenosine receptor antagonist, antineoplastic, 6LSF69F6A8, M1069 , M 1069
Muvadenant is a small molecule drug. The usage of the INN stem ‘-adenant’ in the name indicates that Muvadenant is a adenosin receptor antagonist. Muvadenant has a monoisotopic molecular weight of 430.17 Da.
Adenosine is an ubiguitous modulator of numerous physiological activities, particularly within the cardiovascular, nervous and immune systems. Adenosine is related both structurally and metabolically to the bioactive nucleotides adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP) and cyclic adenosine monophosphate (cAMP), to the biochemical methylating agent S-adenosyl-L-methione (SAM) and structurally to the coenzymes NAD, FAD and coenzym A and to RNA.
Via cell surface receptors, adenosine modulates diverse physiological functions including induction of sedation, vasodilatation, suppression of cardiac rate and contractility, inhibition of platelet aggregability, stimulation of gluconeogenesis and inhibition of lipolysis. Studies show that adenosine is able to activate adenylate cyclases, open potassium channels, reduce flux through calcium channels, and inhibit or stimulate phosphoinositide turnover through receptor-mediated
mechanisms (Muller C. E. and Stein B., Current Pharmaceutical Design, 2: 501 , 1996; Muller C. E., Exp. Opin. Ther. Patents, 7(5): 419, 1997).
Adenosine receptors belong to the superfamily of G-protein-coupled receptors (GPCRs). Four major subtypes of adenosine receptors have been
pharmacologically, structurally and functionally characterized (Fredholm et al., Pharm. Rev., 46: 143-156, 1994) and referred to as A1, A2A, A2B and A3. Though the same adenosine receptor can couple to different G-proteins, adenosine A1 and A3 receptors usually couple to inhibitory G-proteins referred to as G, and Go which inhibit adenylate cyclase and down-regulate cellular cAMP levels. In contrast, the adenosine A2A and A2B receptors couple to stimulatory G-proteins referred to as Gs that activate adenylate cyclase and increase intracellular levels of cAMP (Linden J., Annu. Rev. Pharmacol. Toxicol., 41 : 775-87 2001).
PAT
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: US-2022119412-A1Priority Date: 2019-01-22
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: EP-3914600-B1Priority Date: 2019-01-22Grant Date: 2024-08-07
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: CN-113939520-BPriority Date: 2019-01-22Grant Date: 2024-11-22
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: ES-2992081-T3Priority Date: 2019-01-22Grant Date: 2024-12-09
- Thiazolopyridine derivatives as adenosine receptor antagonists.Publication Number: JP-7600119-B2Priority Date: 2019-01-22Grant Date: 2024-12-16
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: AU-2020211697-A1Priority Date: 2019-01-22
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: KR-20210116572-APriority Date: 2019-01-22
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: CN-113939520-APriority Date: 2019-01-22
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: EP-3914600-A1Priority Date: 2019-01-22
- Thiazolopyridine derivative as an adenosine receptor antagonistPublication Number: JP-2022524914-APriority Date: 2019-01-22
- Combination therapy for cancerPublication Number: CN-117858723-APriority Date: 2021-06-07
- Combination treatment of cancerPublication Number: EP-4351640-A1Priority Date: 2021-06-07
- Combination Treatment of CancerPublication Number: JP-2024520764-APriority Date: 2021-06-07
- Combination Treatment of CancerPublication Number: US-2024279338-A1Priority Date: 2021-06-07
- Thiazolopyridine derivatives as adenosine receptor antagonistsPublication Number: WO-2020152132-A1Priority Date: 2019-01-22
- Novel crystalline forms of (s)-7-oxa-2-aza-spiro[4.5]decane-2-carboxylic acid [7-(3,6-dihydro-2h-pyran-4-yl)-4-methoxy-thiazolo[4,5-c]pyridin-2-yl]-amide and co-crystal forms thereofPublication Number: TW-202415667-APriority Date: 2022-08-02
- Novel crystalline forms of (s)-7-oxa-2-aza-spiro[4.5]decane-2-carboxylic acid [7-(3,6-dihydro-2h-pyran-4-yl)-4-methoxy-thiazolo[4,5-c]pyridin-2-yl]-amide and co-crystal forms thereofPublication Number: WO-2024028273-A1Priority Date: 2022-08-02
- Combination treatment of cancerPublication Number: WO-2022258622-A1Priority Date: 2021-06-07
- Combination treatment of cancerPublication Number: AU-2022288571-A1Priority Date: 2021-06-07
- Combination treatment of cancerPublication Number: CA-3220380-A1Priority Date: 2021-06-07
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020152132&_cid=P10-MHPOEV-06540-1
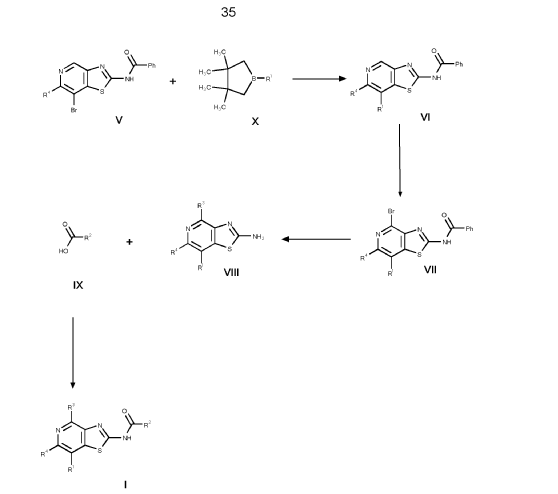
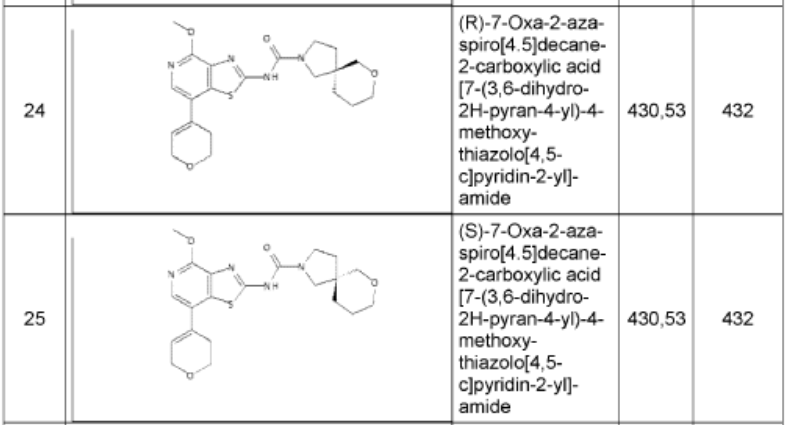
1. (5R)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-thiazolo[4,5-c]pyridin- 2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide 24
and (5S)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-thiazolo[4,5- c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide 25
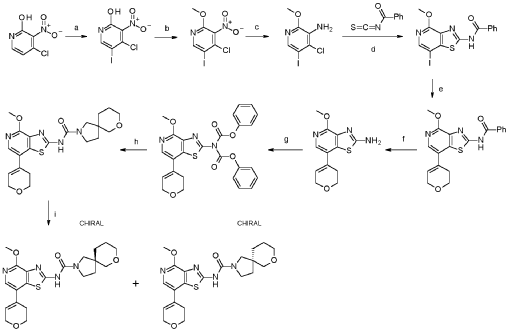
a. 4-chloro-5-iodo-3-nitropyridin-2-ol
Into a 250-mL round-bottom flask was placed 4-chloro-3-nitropyridin-2-ol (10.0 g, 54.4 mmol, 95%), N-lod-succinimid (NIS, 14.2 g, 59.9 mmol, 95%) in acetonitrile (115 mL). The solution was stirred for 1 h overnight at 80°C in an oil bath. The mixture was concentrated and the precipitate formed collected by filtration. The residue was washed with twice with petrol ether (500 mL) dried under vacuum at 60°C overnight. This resulted in 4-chloro-5-iodo-3-nitropyridin-2-ol (16.5 g, 97.9%, 97% purity) as a yellow solid. MS: m/z = 300.9 [M+H]+.
b. 4-chloro-5-iodo-2-methoxy-3-nitropyridine
Into a 500-mL round-bottom flask was placed 4-chloro-5-iodo-3-nitropyridin-2-ol (16.5 g, 53.3 mmol, 97%), Ag2CO3 (15.5 g, 53.3 mmol, 95%) in toluene (310 mL). To this suspension CH3I (15.9 g, 107 mmol, 95%) was added at 50°C and the mixture was stirred at 80°C for 4 h. The precipitate was collected by filtration and discarded. The filtrate was evaporated to dryness under vacuum and the residue purified by silica gel chromatography with ethyl acetate/petroleum ether (15:85).
This resulted in 4-chloro-5-iodo-2-methoxy-3-nitropyridine (9.90 g, 52.6%, 89% purity) as a light yellow solid. MS: m/z = 315.5 [M+H]+.
c. 4-chloro-5-iodo-2-methoxypyridin-3-amine
Into a 250-mL 3-necked round-bottom flask was placed 4-chloro-5-iodo-2-methoxy-3-nitropyridine (9.90 g, 28.0 mmol, 89%), iron (16.5 g, 281 mmol, 95%) and NH 4C (9.40 g, 174 mmol, 99%) in ethanol (152 mL) and water (30 mL). The mixture was stirred for 2 h at 80°C in an oil bath. The reaction mixture was filtered over Celite, washed with ethanol and the mother liquor was concentrated to dryness. The residue was stirred for 30 min. with 100 ml water at 60°dried in vacuo. This resulted in 4-chloro-5-iodo-2-methoxypyridin-3-amine (7.20 g, 75%, 83% purity) as an off-white solid. It was used without further purification in the next step. MS: m/z = 285.9 [M+H]+.
d. N-[7-iodo-4-methoxy-[1,3]thiazolo[4,5-c]pyridin-2-yl]benzamide
Into a 500-mL round-bottom flask was placed 4-chloro-5-iodo-2-methoxypyridin-3-amine (7.20 g, 21.0 mmol, 83%) in acetone (150 mL) and benzoyl isothiocyanate (5.21 g, 31.5 mmol, 99%) was added dropwise at room temperature. The solution was stirred for 1 h at 50 °C in an oil bath. The solids were collected by filtration, washed with acetone and dried in vacuo to give N-[7-iodo-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]benzamide (8.73 g, 91 %, 90% purity) as a white solid. MS: m/z = 412.2 [M+H]+.
e. N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1,3]thiazolo[4,5-c]pyridin- 2-yl]benzamide
To a solution of N-[7-iodo-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]benzamide (6.00 g, 13.1 mmol, 90%) and 2-(3,6-dihydro-2H-pyran-4-yl)-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane (6.13 g, 27.7 mmol, 95%) in dioxane (200 mL) and water (40.00 mL) were added NaOH (2.90 g, 68.9 mmol, 95%) and Pd(dppf)Cl2* dichloromethane (1.20 g, 1.40 mmol, 95%). After stirring for 1 h at 100°C under a nitrogen atmosphere, the mixture was concentrated to dryness under vacuo. The residue was purified by silica gel chromatography with ethyl acetate/hexane (95:5). This resulted in 3.32 g (62%, 90% purity) of N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]benzamide as colorless solid. MS: m/z = 368.1 [M+H]+.
f. 7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1,3]thiazolo[4,5-c]pyridin-2- amine
To a stirred mixture of N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]benzamide (3.27 g, 8.00 mmol, 90%) in water/methanol (1 :1 , 300 mL) was added NaOH (3.36 g, 80.0 mmol, 95%) at room temperature under nitrogen atmosphere. The mixture was stirred for overnight at 90°C under nitrogen atmosphere and evaporated to dryness. The residue was taken up in water and extracted 3 times with dichloromethane (100 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and evaporated to dryness. The residue was purified by silica gel column chromatography, eluted with petrol ether/ethyl acetate (1 :1) to afford 7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-amine (1.50 g, 68%, 96% purity) as a light brownish solid. MS: m/z = 264.1 [M+H]+.
g. phenyl N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy- [1,3]thiazolo[4,5c]pyridin-2-yl]-N-(phenoxycarbonyl)carbamate
To a stirred solution of 7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-amine (600 mg, 2.19 mmol, 96%) and phenyl chloroformate (1.81 g,
11.0 mmol, 95%) in THF (50 mL) was added K2CO3 (1.59 g, 11.0 mmol, 95%) and pyridine (913 mg, 11.0 mmol, 95%) at room temperature under nitrogen
atmosphere. The mixture was stirred for 6 h at 50° and then after re-cooling to room temperature quenched by the addition of water (300 mL). The mixture was extracted 3 times with dichloromethane (200 mL), the combined organic layers were washed once with brine (200 mL), dried over anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure. This resulted in phenyl N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]-N-(phenoxycarbonyl)carbamate (1.00 g, 69%, 76% purity) as a light yellow solid. The crude product was used in the next step directly without further purification. MS: m/z = 504.1 [M+H]+.
h. N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1,3]thiazolo[4,5-c]pyridin- 2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide
To a mixture of phenyl N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]-N-(phenoxycarbonyl)carbamate (1.00 g, 1.52 mmol, 76.) and bis(7-oxa-2-azaspiro[4.5]decane), oxalic acid (1.19 g, 3.03 mmol, 95%) in THF (50 mL) was added diisopropylethyl amine (1.24 g, 9.09 mmol, 95%) at room temperature under nitrogen atmosphere. The mixture was stirred for 1 h at 60°. After re-cooling to room temperature, the mixture was extracted twice with dichloromethane (100 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and evaporated to dryness. The residue was purified by silica gel column chromatography, eluted with petrol ether/ethyl acetate (1 :1) to afford N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide (600 mg, 92%) as a white solid. HPLC: 99.9 % purity, RT = 1.17 min. MS: m/z = 431.1 [M+H]+. 1 H NMR (300 MHz, DMSO-d6) d 1 1.37 (s, 1 H), 7.95 (s, 1 H), 6.25 (s, 1 H), 4.30-4.29 (m, 2H), 3.99 (s, 3H), 3.89 (t, J=5.4Hz, 2H), 3.61-3.29 (m, 8H), 2.55-2.51 (m, 2H), 1.82-1.54 (m, 6H).
i. (5R)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-thiazolo[4,5-c]pyridin- 2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide 24
and (5S)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-thiazolo[4,5- c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide 25
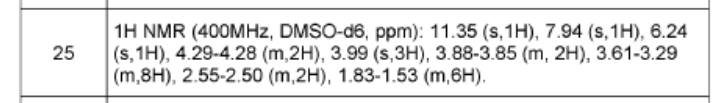
N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide (450 mg, 1.044 mmol, 1 equiv, 99.9%) was purified by chiral-preparative HPLC (Preparative HPLC-032, column: ChiralPak IA, 2*25cm, 5 mm; mobile phase, dichloromethane:ethanol (20:80); detector, UV). This resulted in (5R)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide (178 mg, 39%) as a white solid. HPLC: 99.7 % purity, RT (chiral) = 3.86 min, 100% ee. MS: m/z = 431.2 [M+H]+. 1 H NMR (400 MHz, DMSO-d6) d 1 1.36 (s, 1 H), 7.94 (s, 1 H), 6.24 (s, 1 H), 4.29-4.27 (m, 2H), 3.97 (s,3H), 3.88 (t, J=5.2 Hz, 2H), 3.51-3.19 (m, 8H), 2.55-2.50 (m, 2H), 1.83-1.53 (m, 6H) and (5S)-N-[7-(3,6-dihydro-2H-pyran-4-yl)-4-methoxy-[1 ,3]thiazolo[4,5-c]pyridin-2-yl]-7-oxa-2-azaspiro[4.5]decane-2-carboxamide (171 mg, 38%) as a white solid. HPLC: 99.8 % purity, RT (chiral) = 5.23 min, 99.9% ee. MS: m/z = 431.2 [M+H]+. 1 H NMR (400 MHz, DMSO-d6) d 1 1.35 (s, 1 H), 7.94 (s, 1 H), 6.24 (s, 1 H), 4.29-4.28 (m, 2H), 3.99 (s, 3H), 3.88-3.85 (m, 2H), 3.61-3.29 (m, 8H), 2.55-2.50 (m,2H), 1.83-1.53 (m,6H).
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2024028273&_cid=P10-MHPOFP-06905-1


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
//////////muvadenant, adenosine receptor antagonist, antineoplastic, 6LSF69F6A8, M1069 , M 1069














