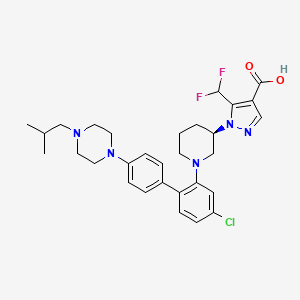
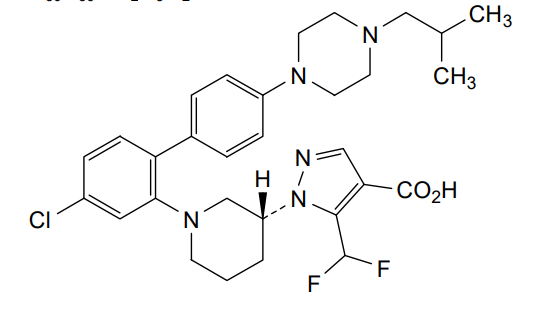
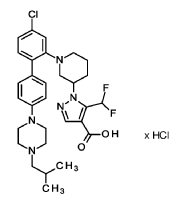
Nurandociguat
CAS 2781965-75-9
MF C30H36ClF2N5O2 MW 572.1 g/mol
1-[(3R)-1-{4-chloro-4′-[4-(2-methylpropyl)piperazin-1-yl][1,1′-biphenyl]-2-yl} piperidin-3-yl]-5-(difluoromethyl)-1H-pyrazole-4-carboxylic acid
1-[(3R)-1-[5-chloro-2-[4-[4-(2-methylpropyl)piperazin-1-yl]phenyl]phenyl]piperidin-3-yl]-5-(difluoromethyl)pyrazole-4-carboxylic acid
guanylate cyclase activator, BAY 3283142, LPU8429UK5
Nurandociguat is a small molecule drug candidate, previously known as BAY 3283142, that is a guanylate cyclase activator being developed by Bayer for cardiovascular conditions. The “ciguat” stem in its name indicates its function as a guanylate cyclase activator, a mechanism that is also being investigated for related drugs like runcaciguat. It is currently in clinical trials, including a Phase 2 program for chronic kidney disease (CKD).
- Drug class: Guanylate cyclase activator
- Developer: Bayer
- Previous name: BAY 3283142
- Indication: Investigated for cardiovascular conditions
- Current status: In clinical development, including a Phase 2 study for chronic kidney disease (CKD)
- OriginatorBayer
- ClassAntihypertensives; Cardiovascular therapies; Hepatoprotectants; Urologics
- Mechanism of ActionGuanylate cyclase stimulants
- Phase IIRenal failure
- Phase ICardiovascular disorders; Diabetic retinopathy; Hypertension; Liver disorders
- 28 Sep 2025No recent reports of development identified for phase-I development in Renal-failure in Germany (PO, Immediate release)
- 16 Sep 2025(CTIS2024-510856-11-00) (EudraCT2024-510856-11-00): Trial initiation and completion info added; updated DevT; Corrected intro to match DevT as most of the info about indication and countries missing
- 28 May 2025No recent reports of development identified for phase-I development in Renal-failure(In volunteers, In adults) in Japan (PO, Immediate release)
Nurandociguat is a small molecule drug. The usage of the INN stem ‘-ciguat’ in the name indicates that Nurandociguat is a guanylate cyclase activator and stimulator. Nurandociguat has a monoisotopic molecular weight of 571.25 Da.
PAT
- Soluble guanylate cyclase activators for use in the treatment of heart failure with preserved ejection fraction in womenPublication Number: WO-2023237577-A1Priority Date: 2022-06-09
- Substituted pyrazolo piperidine carboxylic acidsPublication Number: WO-2022122910-A1Priority Date: 2020-12-10
- The use of sgc activators for the treatment of ophthalmologic diseasesPublication Number: WO-2022122917-A1Priority Date: 2020-12-10
- Use of sGC activators for the treatment of ophthalmic diseasesPublication Number: CN-115175681-APriority Date: 2020-12-10
- Use of sgc activators for the treatment of ophthalmologic diseasesPublication Number: US-2022241273-A1Priority Date: 2020-12-10
- Use of sGC activators for the treatment of ophthalmic diseasesPublication Number: JP-2023514928-APriority Date: 2020-12-10
- The use of sgc activators for the treatment of ophthalmologic diseasesPublication Number: EP-4259140-A1Priority Date: 2020-12-10
- Use of sGC activators for the treatment of ophthalmic diseasesPublication Number: KR-20230118143-APriority Date: 2020-12-10
- Substituted pyrazolo piperidine carboxylic acidsPublication Number: US-2023265072-A1Priority Date: 2020-12-10
- Use of sGC activators for the treatment of ophthalmic diseasesPublication Number: JP-2024073585-APriority Date: 2020-12-10
- Use of sGC activators for the treatment of ophthalmological diseasesPublication Number: JP-7458683-B2Priority Date: 2020-12-10Grant Date: 2024-04-01
- Use of sgc activators for the treatment of ophthalmologic diseasesPublication Number: US-2023346777-A1Priority Date: 2020-12-10
- Use of sGC activators for treating ophthalmic diseasesPublication Number: CN-115175681-BPriority Date: 2020-12-10Grant Date: 2024-10-25
SYN
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022122917&_cid=P20-MHVQYD-96133-1
soluble guanylate cyclase (sGC) activators for use in the treatment and/or prophylaxis of ophthalmologic diseases, including non-proliferative diabetic retinopathy (NPDR), diabetic macular edema (DME), retinal ganglion cell/photoreceptor neurodegeneration and cataract, especially wherein the soluble guanylate cyclase (sGC) activators are compounds selected from the group consisting of
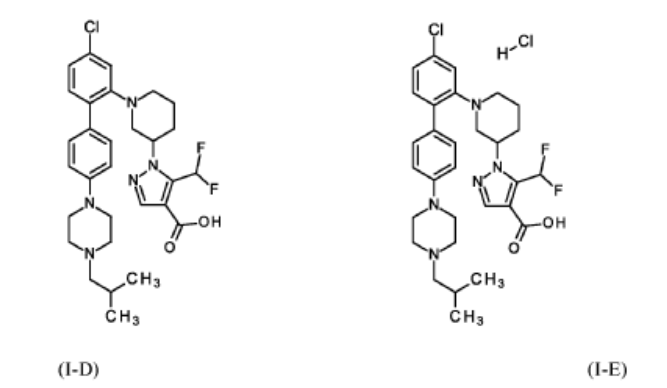
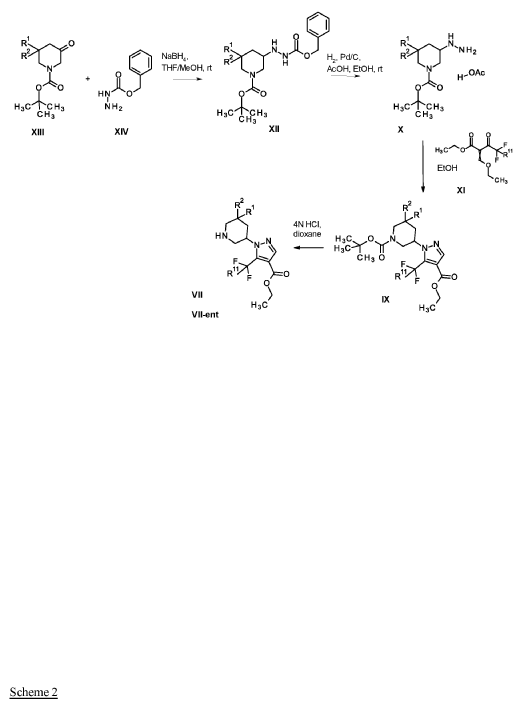
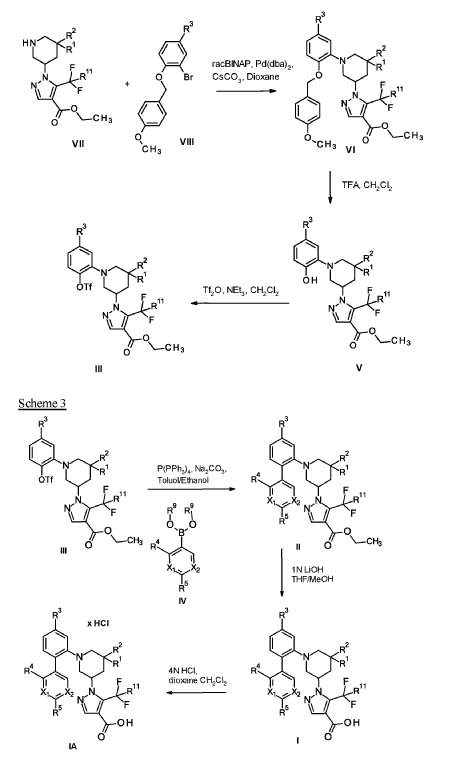
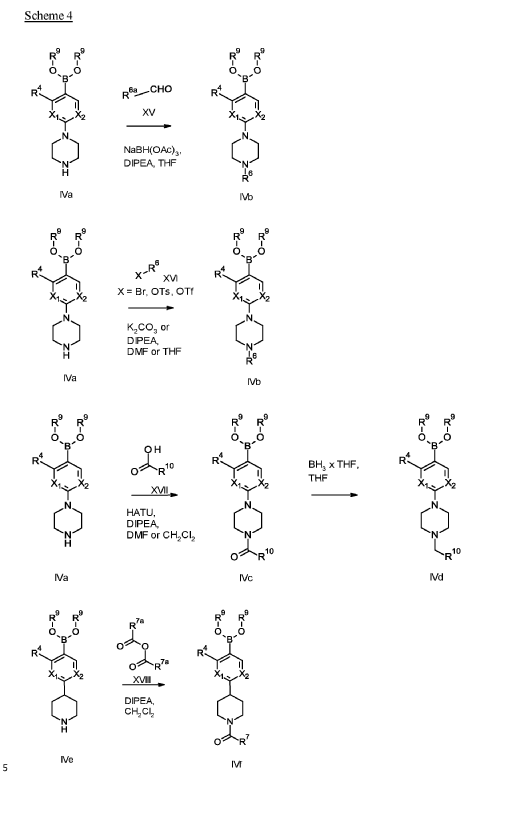
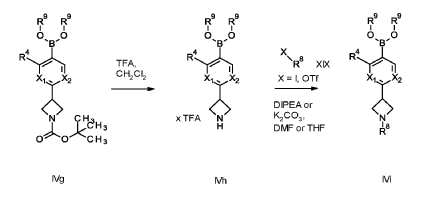
Example 1
1 – [ 1 – { 4-Chloro-4′- [4-(2-methylpropyl)piperazin- 1 -yl] [1,1 ’-biphenyl] -2-yl }piperidin-3-yl] -5- (difluoromethyl)-lH-pyrazole-4-carboxylic acid hydrochloride (Enantiomer 1)

Ethyl 1 – [ 1 – { 5-chloro-2- [(trifluoromethanesulfonyl)oxy]phenyl }piperidin-3-yl] -5-(difluoromethyl)- 1 H-pyrazole-4-carboxylate (prepared in analogy to Example 11A, Enantiomer 1, 80.0 mg, 147 pmol) and l-(2-methylpropyl)-4- [4-(4,4,5 ,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)phenyl]piperazine (Example 18 A 62.8 mg, 97 % purity, 177 pmol) were placed under argon in toluene/ethanol (820/820 pl). 2 M sodium carbonate solution (220 pl, 2.0 M, 440 pmol) and tetrakis(triphenylphosphine)palladium(0) (8.52 mg, 7.37 pmol) were added and the mixture was stirred at 100°C. overnight. The reaction mixture was diluted with ethyl acetate and 1 M hydrochloric acid was added. The aqueous phase was extracted three times with ethyl acetate. The organic phase was dried with sodium sulfate, filtered off and evaporated. The crude mixture was dissolved with THF/ethanol (2.0/0.2 ml), 1 M lithium hydroxide solution (1.5 ml, 1.5 mmol) was added and the mixture was stirred at room temperature overnight. A I M lithium hydroxide solution (740 pl, 740 pmol) was added again. After about 6 h the reaction mixture was evaporated at 50°C. The residue was dissolved in
SUBSTITUTE SHEET (RULE 26)
acetonitrile/water/0.25 ml trifluoroacetic acid and purified by preparative HPLC (RP18 column, acetonitrile/water gradient with the addition of 0.1% trifluoroacetic acid). The crude product was purified by means of thick layer chromatography (dichloromethane/methanol/formic acid: 10/1/0.1). The silica gel mixture was stirred with dichloromethane/1 M hydrochloric acid in dioxane (10/1) in ethanol, filtered off and carefully evaporated at 30°C and lyophilized. 34 mg of the target compound (36% of theory, purity 95%) were obtained.
LC-MS (Method 6): Rt = 1.23 min; MS (ESIpos): m/z = 572 [M-HC1+H]+
‘H-NMR (600 MHz, DMSO-d6) 5 [ppm]: 1.004 (15.87), 1.015 (16.00), 1.500 (0.51), 1.521 (0.57), 1.728 (0.73), 1.750 (0.61), 1.897 (0.57), 1.917 (0.62), 1.975 (0.79), 2.122 (0.42), 2.133 (0.84), 2.144 (1.02), 2.156
(0.79), 2.571 (0.47), 2.587 (0.91), 2.610 (0.52), 3.004 (0.84), 3.022 (2.01), 3.026 (2.20), 3.038 (3.72), 3.048
(2.50), 3.065 (0.75), 3.154 (2.66), 3.161 (2.75), 3.169 (2.36), 3.177 (1.88), 3.224 (0.84), 3.237 (0.70), 3.589
(1.41), 3.602 (1.80), 3.825 (1.02), 3.841 (0.78), 3.866 (1.05), 3.882 (0.75), 4.223 (2.57), 4.445 (0.68), 4.463
(0.97), 4.481 (0.57), 7.045 (0.55), 7.055 (3.63), 7.070 (3.72), 7.084 (2.72), 7.087 (3.09), 7.110 (1.47), 7.113
(1.11), 7.123 (2.19), 7.127 (2.02), 7.163 (3.67), 7.177 (2.19), 7.215 (0.46), 7.428 (0.83), 7.495 (4.24), 7.510
(4.02), 7.515 (2.07), 7.602 (0.82), 7.959 (4.79), 9.484 (0.54).
Example 2
1 – [ 1 – { 4-Chloro-4′- [4-(2-methylpropyl)piperazin- 1 -yl] [1,1 ’-biphenyl] -2-yl }piperidin-3-yl] -5-(difluoromethyl)-lH-pyrazole-4-carboxylic acid (Enantiomer 2)
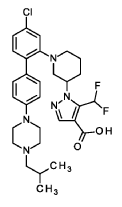
Method A
A solution of ethyl l-[l-{4-chloro-4′-[4-(2-methylpropyl)piperazin-l-yl][l,T-biphenyl]-2-yl}piperidin-3-yl]-5-(difluoromethyl)-lH-pyrazole-4-carboxylate (prepared in analogy to Example 17A, Enantiomer 2, 50.8 g, 84.6 mmol) in a THF/methanol mixture 9:1 (1.0 1) was treated with an aqueous solution of lithium hydroxide (850 ml, 1.0 M, 850 mmol) and stirred overnight at room temperature. The reaction mixture was
SUBSTITUTE SHEET (RULE 26)
concentrated, diluted with dichloromethane (1.5 1) and adjusted to pH = 2 with an aqueous solution of hydrogen chloride (2N). The resulting suspension was stirred 45 minutes at room temperature. The solid was filtered, washed with water and dried under vacuum affording 43 g (90 % yield) of the title compound.
LC-MS (Method 7): Rt = 1.27 min; MS (ESIpos): m/z = 572 [M+H]+
‘H-NMR (600 MHz, DMSO-d6) 5 [ppm]: 1.002 (15.68), 1.013 (16.00), 1.080 (0.57), 1.092 (1.18), 1.103 (0.63), 1.498 (0.74), 1.519 (0.83), 1.719 (1.03), 1.741 (0.88), 1.902 (0.78), 1.908 (0.74), 1.922 (0.88), 1.928
(0.83), 1.943 (0.45), 1.978 (1.13), 1.994 (0.74), 2.102 (0.71), 2.112 (0.85), 2.123 (0.70), 2.571 (1.40), 2.591
(0.77), 2.882 (1.10), 3.018 (1.27), 3.035 (3.01), 3.053 (2.14), 3.239 (2.40), 3.254 (2.32), 3.368 (1.13), 3.379
(1.40), 3.391 (1.33), 3.403 (0.92), 3.493 (0.76), 4.463 (0.65), 4.482 (1.12), 4.500 (0.62), 7.033 (4.22), 7.048
(4.45), 7.074 (3.47), 7.077 (4.04), 7.100 (1.85), 7.103 (1.52), 7.113 (2.53), 7.117 (2.34), 7.162 (4.18), 7.175
(2.71), 7.439 (1.03), 7.481 (4.88), 7.495 (4.57), 7.526 (2.04), 7.613 (0.91), 7.952 (5.28).
Method B
1 – { 1 – [4-Chloro-4′-(4-isobutylpiperazin- 1 -yl) [biphenyl] -2-yl]piperidin-3-yl } -5-(difluoromethyl)- 1 H-pyrazole-4-carboxylic acid hydrochloride (prepared in analogy to Example 3, Enantiomer 2, 31.2 mg, 51.3 pmol) were dissolved in 17 ml of dichloromethane and 1 ml of methanol. The solution was shaken once with 1.5 ml of saturated, aqueous sodium bicarbonate solution. The phases were separated. 5 ml of dichloromethane and 3 ml of methanol were added to the organic phase. The organic phase was then dried over sodium sulfate, filtered, evaporated and purified by preparative HPLC (RP18 column, acetonitrile/water gradient, neutral without acid addition). Product fractions were combined and lyophilized. 22 mg of the target compound (74% of theory) were obtained.
LC-MS (Method 3): Rt = 1.73 min; MS (ESIpos): m/z = 572 [M+H]+
‘H-NMR (600 MHz, DMSO-d6) 5 [ppm]: 0.887 (15.60), 0.898 (16.00), 1.493 (0.64), 1.514 (0.70), 1.695 (0.89), 1.718 (0.74), 1.799 (0.48), 1.811 (0.88), 1.822 (1.12), 1.833 (0.92), 1.844 (0.48), 1.890 (0.68), 1.910
(0.74), 1.977 (0.93), 1.995 (0.62), 2.118 (3.91), 2.130 (3.66), 2.516 (5.14), 3.017 (1.09), 3.035 (2.76), 3.053
(1.94), 3.181 (5.03), 3.185 (5.02), 3.267 (1.53), 4.473 (0.55), 4.491 (0.96), 4.509 (0.54), 6.963 (3.96), 6.977
(4.06), 7.048 (3.13), 7.051 (3.31), 7.081 (1.60), 7.084 (1.26), 7.095 (2.21), 7.098 (1.89), 7.152 (3.52), 7.165
(2.42), 7.434 (4.45), 7.448 (4.50), 7.533 (1.51), 7.621 (0.67), 7.930 (4.14).
Example 3
1 – { 1 – [4-Chloro-4′-(4-isobutylpiperazin- 1 -yl) [biphenyl] -2-yl]piperidin-3-yl } -5-(difluoromethyl)- 1 H-pyrazole-4-carboxylic acid hydrochloride (Enantiomer 2)
SUBSTITUTE SHEET (RULE 26)
Method A
A suspension of 1 – [ 1 – { 4-chloro-4′- [4-(2-methylpropyl)piperazin- 1 -yl] [1,1 ’-biphenyl] -2-yl }piperidin-3-yl] -5-(difluoromethyl)-lH-pyrazole-4-carboxylic acid (prepared in analogy to Example 2, Enantiomer 2, 43.5 g, 76.0 mmol) in diethyl ether (870 ml) was treated with a solution of hydrogen chloride in diethyl ether (84 ml, 1.0 M, 84 mmol). The resulting mixture was stirred overnight at room temperature and evaporated affording 46.1 g (quant.) of the title compound.
LC-MS (Method 3): Rt = 1.72 min; MS (ESIpos): m/z = 572 [M+H]+
‘H-NMR (600 MHz, DMSO-d6) 5 [ppm]: 1.026 (15.64), 1.037 (16.00), 1.497 (0.56), 1.519 (0.61), 1.722 (0.78), 1.743 (0.65), 1.903 (0.59), 1.910 (0.53), 1.924 (0.66), 1.930 (0.61), 1.978 (0.82), 1.994 (0.50), 2.142
(0.45), 2.154 (0.91), 2.165 (1.11), 2.176 (0.89), 2.187 (0.45), 2.557 (0.64), 2.577 (1.02), 2.594 (0.55), 2.992
(1.81), 3.002 (2.77), 3.012 (1.87), 3.018 (1.15), 3.036 (2.40), 3.054 (1.60), 3.133 (1.12), 3.148 (1.19), 3.168
(0.53), 3.237 (0.88), 3.250 (0.76), 3.338 (0.81), 3.360 (1.42), 3.379 (0.88), 3.580 (1.61), 3.791 (0.89), 3.819
(1.25), 3.844 (0.81), 4.463 (0.89), 4.474 (0.97), 4.481 (1.26), 4.488 (0.99), 4.499 (0.88), 7.051 (3.56), 7.065
(3.77), 7.077 (2.72), 7.080 (3.14), 7.103 (1.42), 7.106 (1.13), 7.116 (2.00), 7.120 (1.84), 7.165 (3.40), 7.178
(2.22), 7.443 (0.84), 7.489 (4.04), 7.504 (3.79), 7.531 (1.66), 7.618 (0.72), 7.954 (4.33), 10.519 (0.49).
Method B
Ethyl 1 – [ 1 – { 5-chloro-2- [(trifluoromethanesulfonyl)oxy]phenyl }piperidin-3-yl] -5-(difluoromethyl)- 1 H-pyrazole-4-carboxylate (prepared in analogy to Example 14A, Enantiomer 2, 80.0 mg, 150 pmol) and l-(2-methylpropyl)-4- [4-(4,4,5 ,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)phenyl]piperazine (Example 18 A 64.1 mg, 97 % purity, 180 pmol) were dissolved under argon in toluene/ethanol (0.83/0.83 ml). Tetrakis(triphenylphosphine)palladium(0) (8.69 mg, 7.52 pmol) and 2 M sodium carbonate solution (226 pl, 452 pmol) were added and the mixture was stirred at 100°C overnight. The reaction mixture was diluted with ethyl acetate and water. The aqueous phase was acidified with 1 M hydrochloric acid. The phases were
SUBSTITUTE SHEET (RULE 26)
separated and the aqueous phase was extracted twice with ethyl acetate. The combined organic phases were dried over sodium sulfate, filtered and evaporated. The crude product was dissolved in THF/ethanol (3.9/0.39 ml), 1 M aqueous lithium hydroxide solution (1.5 ml, 1.5 mmol) was added and the mixture was stirred overnight at room temperature. The mixture was evaporated, the residue was dissolved in acetonitrile/TFA/water and purified using preparative HPLC (RP18 column, acetonitrile/water gradient with the addition of 0.1% TFA). The product fractions were combined and evaporated. The residue was mixed with 0.1 M hydrochloric acid in dioxane, carefully evaporated at 30°C (twice) and then lyophilized. 53 mg of the target compound (55% of theory, purity 95%) were obtained.
LC-MS (Method 4): Rt = 0.91 min; MS (ESIpos): m/z = 572 [M-HC1+H]+
‘H-NMR (400 MHz, DMSO-d6) 5 [ppm]: 1.004 (15.46), 1.020 (16.00), 1.491 (0.44), 1.522 (0.50), 1.722 (0.68), 1.753 (0.55), 1.890 (0.47), 1.920 (0.55), 1.967 (0.84), 2.129 (0.76), 2.146 (0.96), 2.163 (0.76), 2.582
(0.91), 2.613 (0.48), 2.999 (0.86), 3.010 (1.71), 3.025 (3.88), 3.041 (2.30), 3.131 (0.88), 3.161 (1.25), 3.177
(2.08), 3.213 (1.75), 3.242 (1.16), 3.467 (1.06), 3.496 (0.84), 3.503 (0.60), 3.519 (0.54), 3.525 (0.50), 3.549
(0.75), 3.555 (0.84), 3.572 (1.57), 3.582 (1.48), 3.589 (1.38), 3.601 (2.78), 3.608 (1.89), 3.633 (0.44), 3.640
(0.41), 3.811 (0.94), 3.847 (1.32), 3.878 (0.71), 4.329 (0.49), 4.439 (0.46), 4.466 (0.73), 4.477 (0.52), 4.839
(0.49), 7.047 (3.30), 7.070 (3.64), 7.082 (2.61), 7.087 (3.29), 7.104 (1.46), 7.109 (0.86), 7.124 (2.34), 7.129
(2.03), 7.160 (3.99), 7.181 (1.96), 7.388 (0.88), 7.490 (4.02), 7.512 (3.81), 7.519 (2.20), 7.650 (0.72), 7.959
(3.78), 9.708 (0.41).
[OC]D20 = -73.05°, c = 0.465g/100cm3, trichloromethane.
Enantiomer 2 has an absolute configuration of R as shown in example 3 A below.
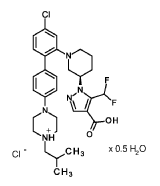
1 – { 3(2?)- 1 – [4-Chloro-4′-(4-isobutylpiperazin- 1 -yl) [biphenyl] -2-yl]piperidin-3-yl } -5-(difluoromethyl)- 1H-pyrazole-4-carboxylic acid hydrochloride
Example 3A
1 – { 3(7?)- 1 – [4-Chloro-4′-(4-isobutylpiperazin- 1 -yl) [biphenyl] -2-yl]piperidin-3-yl } -5-(difluoromethyl)- 1H-pyrazole-4-carboxylic acid hydrochloride hemihydrate
SUBSTITUTE SHEET (RULE 26)
100 mg 1 – { 1 – [4-Chloro-4′-(4-isobutylpiperazin- 1 -yl) [biphenyl] -2-yl]piperidin-3-yl } -5-(difluoromethyl)-lH-pyrazole-4-carboxylic acid hydrochloride (Enantiomer 2) (example 3) were solved at 60°C in 3,5 ml 2 -propanol, wherein the 2-propanol was dosed portion wise in lOOpl -portions at 60°C until a clear solution was obtained. Afterwards the vessel was closed with a septum and placed into a slowly cooling sand bath from 60°C to roomtemperature over the weekend -> small amounts of solids were detected. Thereafter the septum was provided with a canula, in order to slowly let the solvent evaporate. After 4 weeks crystals were collected and inspected under a microscope.



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
/////////Nurandociguat, guanylate cyclase activator, BAY 3283142, LPU8429UK5














