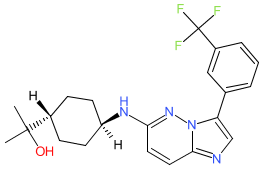
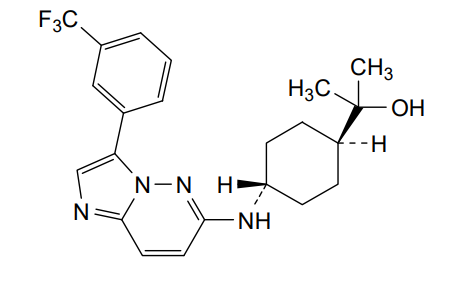

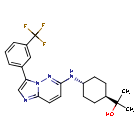
Nuvisertib
CAS 1361951-15-6
MF C22H26ClF3N4O MW418.5 g/mol
2-[(1r,4r)-4-({3-[3-(trifluoromethyl)phenyl]imidazo[1,2-b]pyridazin-6-yl}amino)cyclohexyl]propan-2-ol
serine/ threonine kinase inhibitor, antineoplastic, Orphan Drug, myelofibrosis, SGI-9481, SGI 9481, TP-3654, TP 3654, EOB0N7BOY4
The chemical structure for nuvisertib was obtained from proposed INN list 130 (Feb. 2024), in which the compound is described as a serine/ threonine kinase inhibitor with antineoplastic action. A structure match to clinical lead TP-3654 was made via PubChem. TP-3654 is declared as an orally available, second-generation pan-PIM kinase inhibitor [1-2].
| References |
| 1. Foulks JM, Carpenter KJ, Luo B, Xu Y, Senina A, Nix R, Chan A, Clifford A, Wilkes M, Vollmer D et al.. (2014) A small-molecule inhibitor of PIM kinases as a potential treatment for urothelial carcinomas. Neoplasia, 16 (5): 403-12. [PMID:24953177] |
| 2. Wu CP, Li YQ, Chi YC, Huang YH, Hung TH, Wu YS. (2021) The Second-Generation PIM Kinase Inhibitor TP-3654 Resensitizes ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Cytotoxic Anticancer Drugs. Int J Mol Sci, 22 (17). [PMID:34502348] |
Nuvisertib is an orally available, second-generation and selective ATP-competitive inhibitor of proviral integration site for Moloney murine leukemia virus (PIM) kinases, with potential antineoplastic activity. Upon oral administration, nuvisertib selectively binds to and prevents the activation of the PIM kinases. This prevents the activation of PIM-mediated signaling pathways and inhibits proliferation in cells that overexpress PIM. PIMs, constitutively active proto-oncogenic serine/threonine kinases, are upregulated in various types of cancers and play key roles in tumor cell proliferation and survival.
Nuvisertib, also known as TP-3654, is an oral, investigational, and highly selective PIM1 kinase inhibitor being studied in a Phase 1/2 clinical trial for intermediate- or high-risk myelofibrosis (MF). It is not currently an approved medication.
Key Information
- Mechanism of Action: Nuvisertib targets the PIM1 kinase pathway, which is often overactive in myelofibrosis and can promote cancer cell growth. By inhibiting this pathway, nuvisertib is being investigated for its potential to manage symptoms, reduce spleen size, improve blood counts, and slow the progression of bone marrow fibrosis.
- Current Status: Nuvisertib is in ongoing Phase 1/2 clinical trials (NCT04176198) as a monotherapy and in combination with JAK inhibitors like ruxolitinib and momelotinib.
- Designations: Nuvisertib has received Orphan Drug Designation for myelofibrosis
Study of TP-3654 in Patients With Advanced Solid Tumors
CTID: NCT03715504
Phase: Phase 1
Status: Completed
Date: 2023-11-14
SYN
WO2013013188
Example 31
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US427659372&_cid=P10-MHWTVL-76212-1
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US130491286&_cid=P10-MHWU33-81462-1
31. 4-((3-(3-(Trifluoromethyl)phenyl)imidazo[1,2-b]pyridazin-6-yl)amino)-trans-cyclohexyl)propan-2-ol (EX. 8-31)
| EX. 8-31 was prepared by similar procedures as in EX. 8-1 using 2-(trans-4-aminocyclohexyl)propan-2-ol. |

| 1H-NMR (CD 3OD/400 MHz): δ 8.82 (s, 1H), 8.19 (m, 1H), 7.88 (s, 1H), 7.62 (m, 3H), 6.70 (d, J=9.6 Hz, 1H), 3.71 (m, 1H), 2.26 (m, 2H), 1.95 (m, 2H), 1.36 (m, 1H), 1.27 (m, 4H), 1.21 (s, 6H). MS (ES +, m/z): (M+H) +: 419.6. |
| To a solution of trans-4-((tert-butoxycarbonyl)amino)cyclohexanecarboxylic acid (823 g, 3.38 mol) in EtOAc (4000 mL) was added EA/HCl (2500 mL). The mixture was stirred at 0° C. overnight. The reaction mixture was filtered and dried in vacuo to give a product of hydrochloride salt of trans-4-aminocyclohexanecarboxylic acid as white solid (604 g, 99.42% yield). |
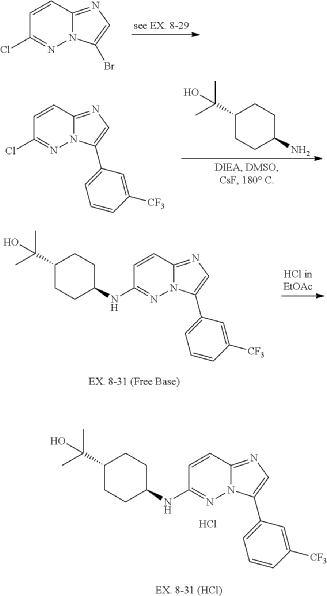
| 6-chloro-3-(3-(trifluoromethyl)phenyl)imidazo[1,2-b]pyridazine was prepared according to procedure in EX. 8-29. |
PAT
- Heterocyclic protein kinase inhibitorsPublication Number: ES-2834093-T3Priority Date: 2011-07-21Grant Date: 2021-06-16
- Substituted imidazo[1,2-b]pyridazines as protein kinase inhibitorsPublication Number: US-2021238183-A1Priority Date: 2011-07-21
- Imidazo[1,2-b]pyridazine and pyrazolo[1,5-a]pyrimidine derivatives and their use as protein kinase inhibitorsPublication Number: US-2012058997-A1Priority Date: 2006-11-06
- Substituted imidazo[1,2-b]pyridazines as protein kinase inhibitorsPublication Number: US-9416132-B2Priority Date: 2011-07-21Grant Date: 2016-08-16
- Heterocyclic protein kinase inhibitorsPublication Number: WO-2013013188-A1Priority Date: 2011-07-21
- Heterocyclic protein kinase inhibitorsPublication Number: EP-3409278-B1Priority Date: 2011-07-21Grant Date: 2020-09-16
- Substituted imidazo[1,2-B]pyridazines as protein kinase inhibitorsPublication Number: US-10875864-B2Priority Date: 2011-07-21Grant Date: 2020-12-29
- Heterocyclic protein kinase inhibitorsPublication Number: EP-3812387-A1Priority Date: 2011-07-21
- Substituted imidazo[1,2-B]pyridazines as protein kinase inhibitorsPublication Number: US-10392392-B2Priority Date: 2011-07-21Grant Date: 2019-08-27
- Heterocyclic protein kinase inhibitorsPublication Number: US-2014329807-A1Priority Date: 2011-07-21
- Substituted imidazo[1,2-b]pyridazines as protein kinase inhibitorsPublication Number: US-2017002014-A1Priority Date: 2011-07-21
- Substituted imidazo[1,2-b]pyridazines as protein kinase inhibitorsPublication Number: US-2019071446-A1Priority Date: 2011-07-21
- Substituted imidazo[1,2-b]pyridazines as protein kinase inhibitorsPublication Number: US-2020102313-A1Priority Date: 2011-07-21
- Heterocyclic protein kinase inhibitorsPublication Number: EP-2734205-B1Priority Date: 2011-07-21Grant Date: 2018-03-21
- Heterocyclic protein kinase inhibitorsPublication Number: EP-3409278-A1Priority Date: 2011-07-21
- Heterocyclic protein kinase inhibitorsPublication Number: JP-2014520898-APriority Date: 2011-07-21
- Heterocyclic protein kinase inhibitorsPublication Number: JP-6105578-B2Priority Date: 2011-07-21Grant Date: 2017-03-29
- Substituted imidazo[1,2-B]pyridazines as protein kinase inhibitorsPublication Number: US-10047093-B2Priority Date: 2011-07-21Grant Date: 2018-08-14


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
REF
– Nuvisertib (TP-3654), an investigational highly selective oral PIM1 kinase inhibitor, is being evaluated in patients with relapsed or refractory myelofibrosis (MF) –
– Nuvisertib demonstrated symptom and spleen responses correlating with cytokine modulation in the preliminary Phase 1/2 data recently presented at the European Hematology Association (EHA) 2025 Congress –
MARLBOROUGH, Mass., June 12, 2025 /PRNewswire/ — Sumitomo Pharma America, Inc. (SMPA) today announced that the U.S. Food and Drug Administration (FDA) granted Fast Track Designation to nuvisertib (TP-3654) for the treatment of patients with intermediate or high-risk myelofibrosis (MF). The FDA Fast Track Designation is granted to investigational therapies being developed to treat serious or life-threatening conditions that demonstrate the potential to address unmet medical needs. Nuvisertib is an oral, investigational, highly selective inhibitor of PIM1 kinase, which demonstrated clinical activity including symptom and spleen responses correlating with cytokine modulation in the updated preliminary Phase 1/2 data presented at the European Hematology Association (EHA) 2025 Congress in Milan, Italy.
MF, a serious and rare type of blood cancer, is characterized by the buildup of fibrous tissues in the bone marrow which is caused by dysregulation in the Janus-associated kinase (JAK) signaling pathway. The clinical manifestations of MF include an enlarged spleen, debilitating symptoms and reduction in hemoglobin and/or platelets. MF affects 1 in 500,000 people worldwide.1
“This positive momentum for nuvisertib signals strong promise in our pipeline and reflects our dedication to addressing unmet medical needs on behalf of patients with myelofibrosis and their families,” said Tsutomu Nakagawa, Ph.D, President and Chief Executive Officer of SMPA. “Receiving FDA Fast Track Designation for nuvisertib in the treatment of myelofibrosis reinforces our confidence in its potential as a treatment option for patients facing a poor prognosis with limited treatment options. We are committed to working closely with the FDA to progress the clinical development of nuvisertib and bring an alternative treatment option to patients with myelofibrosis.”
Updated data from the ongoing Phase 1/2 study of nuvisertib in patients with relapsed/refractory MF were presented at the EHA Congress on June 12, 2025. Preliminary data showed that nuvisertib monotherapy appears to be well tolerated with no dose-limiting toxicities (DLTs). Evaluable patients showed clinical activity including a ≥25% spleen volume reduction (SVR25) in 22.2% of patients and a ≥50% reduction in total symptom score (TSS50) of 44.4% of patients, as well as improvement of bone marrow fibrosis (42.9% patients), hemoglobin (24% patients) and platelet count (26.7% patients). Data also showed that nuvisertib treatment led to significant cytokine modulation [reduction of pro-inflammatory cytokines (e.g. EN-RAGE, MIP-1β) and increase of anti-inflammatory cytokines (e.g. adiponectin)], which demonstrated significant (p<0.001) correlation with symptom and spleen responses. Preclinical2 and emerging clinical data support the development of nuvisertib in combination with JAK inhibitors for the treatment of patients with MF.
“The data observed to date demonstrate promising clinical activity for nuvisertib and the strong potential for selective PIM1 inhibition to slow the progression of myelofibrosis,” said Jatin Shah, MD, Chief Medical Officer, Oncology. “Patients with myelofibrosis are in need of new therapeutic approaches, including combination treatment options, that can provide increased and durable response rates with limited hematologic adverse events. The FDA Fast Track Designation reinforces the potential of nuvisertib to provide clinical benefits for patients with myelofibrosis, an unmet medical need.”
About Nuvisertib (TP-3654)
Nuvisertib (TP-3654) is an oral investigational selective inhibitor of PIM1 kinase, which has shown potential antitumor and antifibrotic activity through multiple pathways, including induction of apoptosis in preclinical models.2,3 Nuvisertib was observed to inhibit proliferation and increase apoptosis in murine and human hematopoietic cells expressing the clinically relevant JAK2 V617F mutation.3 Nuvisertib alone and in combination with ruxolitinib showed white blood cell and neutrophil count normalization, and also reduced spleen size and bone marrow fibrosis in JAK2 V617F and MPLW515L murine models of myelofibrosis.2 The safety and efficacy of nuvisertib is currently being clinically evaluated in a Phase 1/2 study in patients with intermediate and high-risk myelofibrosis (NCT04176198). The U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation to nuvisertib for the indication of myelofibrosis in May 2022. The Japan Ministry of Health, Labour and Welfare (MHLW) granted Orphan Drug Designation to nuvisertib for the treatment of myelofibrosis in November 2024.
About Sumitomo Pharma
Sumitomo Pharma Co., Ltd., is a global pharmaceutical company based in Japan with key operations in the U.S. (Sumitomo Pharma America, Inc.), Canada (Sumitomo Pharma Canada, Inc.), and Europe (Sumitomo Pharma Switzerland GmbH) focused on addressing patient needs in oncology, urology, women’s health, rare diseases, psychiatry & neurology, and cell & gene therapies. With several marketed products in the U.S., Canada, and Europe, a diverse pipeline of early- to late-stage assets, we aim to accelerate discovery, research, and development to bring novel therapies to patients sooner. For more information on SMPA, visit our website https://www.us.sumitomo-pharma.com or follow us on LinkedIn.
The Sumitomo corporate symbol mark is a trademark of Sumitomo Pharma Co., Ltd., used under license. SUMITOMO PHARMA is a trademark of Sumitomo Pharma Co., Ltd., used under license. SUMITOMO is a registered trademark of Sumitomo Chemical Co., Ltd., used under license. Sumitomo Pharma America, Inc. is a U.S. subsidiary of Sumitomo Pharma Co., Ltd.
©2025 Sumitomo Pharma America, Inc. All rights reserved.
References
- U.S. National Library of Medicine. (n.d.). Primary myelofibrosis: Medlineplus Genetics. MedlinePlus. https://medlineplus.gov/genetics/condition/primary-myelofibrosis/
- Dutta A., Nath D, Yang Y, et al. Genetic ablation of Pim1 or pharmacologic inhibition with TP-3654 ameliorates myelofibrosis in murine models. Leukemia. 2022; 36 (3): 746-759. doi: 10.1038/s41375-021-01464-2.
- Foulks JM, Carpenter KJ, Luo B, et al. A small-molecule inhibitor of PIM kinases as a potential treatment for urothelial carcinomas. Neoplasia. 2014;16(5):403-412.
SOURCE Sumitomo Pharma America
- BLM overexpression as a predictive biomarker for CHK1 inhibitor response in PARP inhibitor–resistant BRCA -mutant ovarian cancerPublication Name: Science Translational MedicinePublication Date: 2023-06-21PMCID: PMC10758289PMID: 37343085DOI: 10.1126/scitranslmed.add7872
- The Second-Generation PIM Kinase Inhibitor TP-3654 Resensitizes ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Cytotoxic Anticancer DrugsPublication Name: International Journal of Molecular SciencesPublication Date: 2021-08-30PMCID: PMC8431370PMID: 34502348DOI: 10.3390/ijms22179440
- High-Throughput Screening to Identify Inhibitors of the Type I Interferon–Major Histocompatibility Complex Class I Pathway in Skeletal MusclePublication Name: ACS Chemical BiologyPublication Date: 2020-05-27PMCID: PMC7859889PMID: 32459468DOI: 10.1021/acschembio.0c00343
- PIM kinase inhibitors: Structural and pharmacological perspectivesPublication Name: European Journal of Medicinal ChemistryPublication Date: 2019-06-15PMID: 30954777DOI: 10.1016/j.ejmech.2019.03.050
- A Small-Molecule Inhibitor of PIM Kinases as a Potential Treatment for Urothelial CarcinomasPublication Name: Neoplasia (New York, N.Y.)Publication Date: 2014-05PMCID: PMC4198696PMID: 24953177DOI: 10.1016/j.neo.2014.05.004
- BLM overexpression as a predictive biomarker for CHK1 inhibitor response in PARP inhibitor–resistant BRCA -mutant ovarian cancerPublication Name: Science Translational MedicinePublication Date: 2023-06-21PMCID: PMC10758289PMID: 37343085DOI: 10.1126/scitranslmed.add7872
- The Second-Generation PIM Kinase Inhibitor TP-3654 Resensitizes ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Cytotoxic Anticancer DrugsPublication Name: International Journal of Molecular SciencesPublication Date: 2021-08-30PMCID: PMC8431370PMID: 34502348DOI: 10.3390/ijms22179440
- High-Throughput Screening to Identify Inhibitors of the Type I Interferon–Major Histocompatibility Complex Class I Pathway in Skeletal MusclePublication Name: ACS Chemical BiologyPublication Date: 2020-05-27PMCID: PMC7859889PMID: 32459468DOI: 10.1021/acschembio.0c00343
- PIM kinase inhibitors: Structural and pharmacological perspectivesPublication Name: European Journal of Medicinal ChemistryPublication Date: 2019-06-15PMID: 30954777DOI: 10.1016/j.ejmech.2019.03.050
- A Small-Molecule Inhibitor of PIM Kinases as a Potential Treatment for Urothelial CarcinomasPublication Name: Neoplasia (New York, N.Y.)Publication Date: 2014-05PMCID: PMC4198696PMID: 24953177DOI: 10.1016/j.neo.2014.05.004
///////Nuvisertib, serine/ threonine kinase inhibitor, antineoplastic, Orphan Drug, myelofibrosis, SGI-9481, SGI 9481, TP-3654, TP 3654, EOB0N7BOY4














