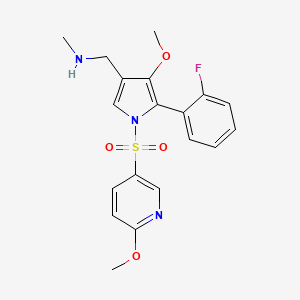
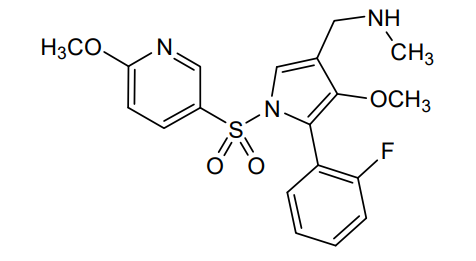
Padoprazan
CAS 2756367-23-2
MF C19H20FN3O4S MW 405.4 g/mol
1-[5-(2-fluorophenyl)-4-methoxy-1-(6-methoxypyridine-3-sulfonyl)-1Hpyrrol-3-yl]-N-methyl methanamine
1-[5-(2-fluorophenyl)-4-methoxy-1-[(6-methoxy-3-pyridinyl)sulfonyl]pyrrol-3-yl]-N-methylmethanamine
1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)- N -methylmethanamine
proton pump inhibitor, 95BJ28E2RP, ID-120040002, ID 120040002
Padoprazan is a new-generation potassium-competitive acid blocker (P-CAB) used to treat acid-related disorders like gastroesophageal reflux, according to MedchemExpress.com and Patsnap Synapse. It works by inhibiting the proton pump in the stomach and is different from traditional proton pump inhibitors (PPIs) because it is not dependent on acid activation. Padoprazan is currently undergoing Phase 3 clinical trials in Korea, notes THE BIO (더바이오).
Key facts about padoprazan
- Drug class: Potassium-competitive acid blocker (P-CAB), a type of proton pump inhibitor, according to DrugBank and GlpBio.
- Mechanism: It inhibits the proton pump in the stomach to reduce acid production and is not acid-activated like older PPIs, per DrugBank.
- Indications: Used for acid-related conditions like gastroesophageal reflux, reports Patsnap Synapse.
- Status: Currently undergoing Phase 3 clinical trials in Korea, says THE BIO (더바이오).
- Development: It is a new-generation drug being developed by companies like Daewon Pharmaceutical.
Padoprazan is a small molecule drug. The usage of the INN stem ‘-prazan’ in the name indicates that Padoprazan is a proton pump inhibitor, not dependent on acid activation. Padoprazan has a monoisotopic molecular weight of 405.12 Da.
PAT
- NEW INHIBITOR OF ACID SECRETION AND USE OF THE SAMEPublication Number: PE-20231652-A1Priority Date: 2020-06-17
- Novel acid secretion inhibitors and use thereofPublication Number: TW-I839161-BPriority Date: 2020-06-17Grant Date: 2024-04-11
- Novel acid secretion inhibitor and use thereofPublication Number: US-2023373954-A1Priority Date: 2020-06-17
- Novel acid secretion inhibitor and use thereofPublication Number: EP-4148050-B1Priority Date: 2020-06-17Grant Date: 2024-12-18
- Novel acid secretion inhibitors and use thereofPublication Number: TW-I797645-BPriority Date: 2020-06-17Grant Date: 2023-04-01
- Acid secretion inhibitor and use thereofPublication Number: US-11767311-B2Priority Date: 2020-06-17Grant Date: 2023-09-26
- Novel Acid Secretion Inhibitor and use thereofPublication Number: AU-2021293694-B2Priority Date: 2020-06-17Grant Date: 2023-12-21
- Novel acid secretion inhibitor and use thereofPublication Number: CN-115884968-BPriority Date: 2020-06-17Grant Date: 2024-06-21
- Novel acid secretion inhibitors and their usesPublication Number: JP-7404561-B2Priority Date: 2020-06-17Grant Date: 2023-12-25
- Novel acid secretion inhibitors and use thereofPublication Number: KR-102432523-B1Priority Date: 2020-06-17Grant Date: 2022-08-16
- Novel acid secretion inhibitor and use thereofPublication Number: CN-115884968-APriority Date: 2020-06-17
- Novel acid secretion inhibitor and use thereofPublication Number: JP-2023524172-APriority Date: 2020-06-17
- Novel acid secretion inhibitor and use thereofPublication Number: US-2023192650-A1Priority Date: 2020-06-17
- Novel acid secretion inhibitors and use thereofPublication Number: TW-202325702-APriority Date: 2020-06-17
- Novel acid secretion inhibitor and use thereofPublication Number: WO-2021256861-A1Priority Date: 2020-06-17
- Novel acid secretion inhibitors and use thereofPublication Number: TW-202214588-APriority Date: 2020-06-17
- Novel Acid Secretion Inhibitor and use thereofPublication Number: AU-2021293694-A1Priority Date: 2020-06-17
- Novel acid secretion inhibitor and use thereofPublication Number: CA-3182882-A1Priority Date: 2020-06-17
- Novel acid secretion inhibitor and use thereofPublication Number: EP-4148050-A1Priority Date: 2020-06-17
- Novel salt of 1-sulfonyl pyrrole derivative, preparation method thereof and pharmaceutical composition comprising thereofPublication Number: TW-I828476-BPriority Date: 2021-12-15Grant Date: 2024-01-01
- Novel salt of 1-sulfonyl pyrrole derivative, preparation method thereof and pharmaceutical composition comprising thereofPublication Number: WO-2023113458-A1Priority Date: 2021-12-15
- Novel salt of 1-sulfonyl pyrrole derivative, method for preparing same, and pharmaceutical composition including samePublication Number: WO-2023113474-A1Priority Date: 2021-12-15
- Novel salt of 1-sulfonyl pyrrole derivative, method for preparing same, and pharmaceutical composition including samePublication Number: US-2025042872-A1Priority Date: 2021-12-15
- Novel acid secretion inhibitors and use thereofPublication Number: KR-20210156234-APriority Date: 2020-06-17
- Novel salt of 1-sulfonylpyrrole derivative, preparation method thereof and pharmaceutical composition comprising the samePublication Number: CN-118541361-APriority Date: 2021-12-15
- Novel salt of 1-sulfonyl pyrrole derivative, preparation method thereof and pharmaceutical composition comprising thereofPublication Number: KR-20230091056-APriority Date: 2021-12-15
- Novel salts of 1-sulfonyl pyrrole derivatives, methods for producing the same, and pharmaceutical compositions containing the samePublication Number: KR-20240119083-APriority Date: 2021-12-15
- Novel salt of 1-sulfonyl pyrrole derivative, preparation method thereof and pharmaceutical composition comprising thereofPublication Number: TW-202334114-APriority Date: 2021-12-15
- Novel formulation comprising acid secretion inhibitorsPublication Number: KR-20240161598-APriority Date: 2023-05-04
- Method for preparation of 6-methoxypyridine-3-yl derivativesPublication Number: TW-202411216-APriority Date: 2022-05-23
- Method for preparing 6-methoxypyridin-3-yl derivativesPublication Number: WO-2023229322-A1Priority Date: 2022-05-23
- Method for preparation of 6-methoxypyridine-3-yl derivativesPublication Number: KR-20230163283-APriority Date: 2022-05-23
- NOVEL SALT OF A DERIVATIVE OF 1-SULFONYLPYRROL, METHOD OF PREPARATION THEREOF AND PHARMACEUTICAL COMPOSITION THAT INCLUDES THE SAMEPublication Number: AR-127964-A1Priority Date: 2021-12-15
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021256861&_cid=P22-MI13VU-05837-1
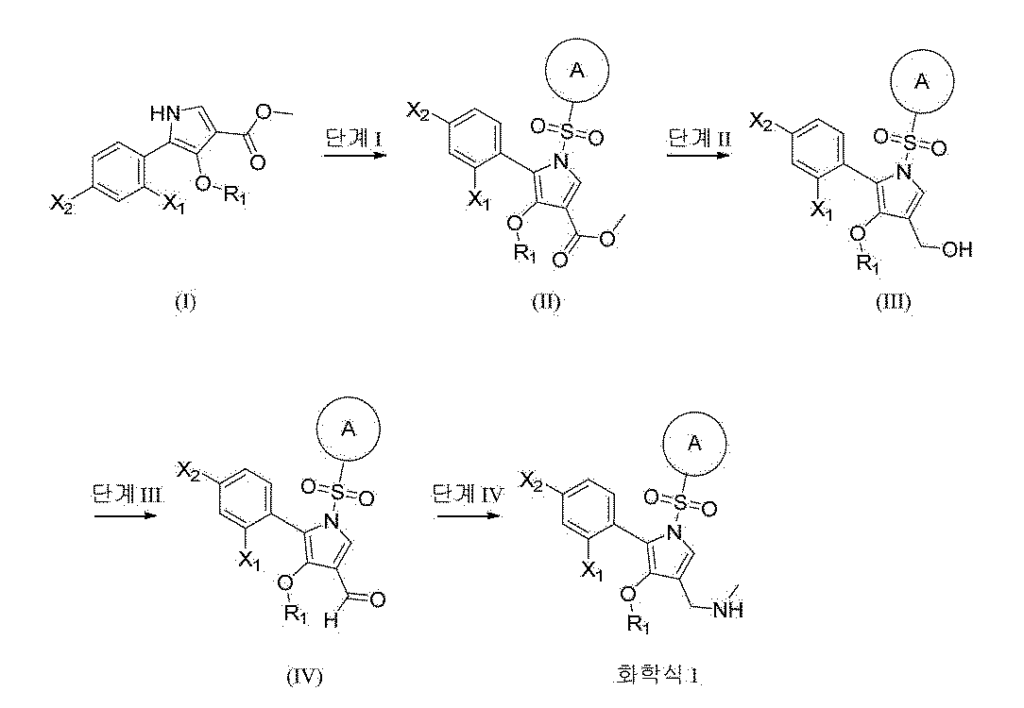
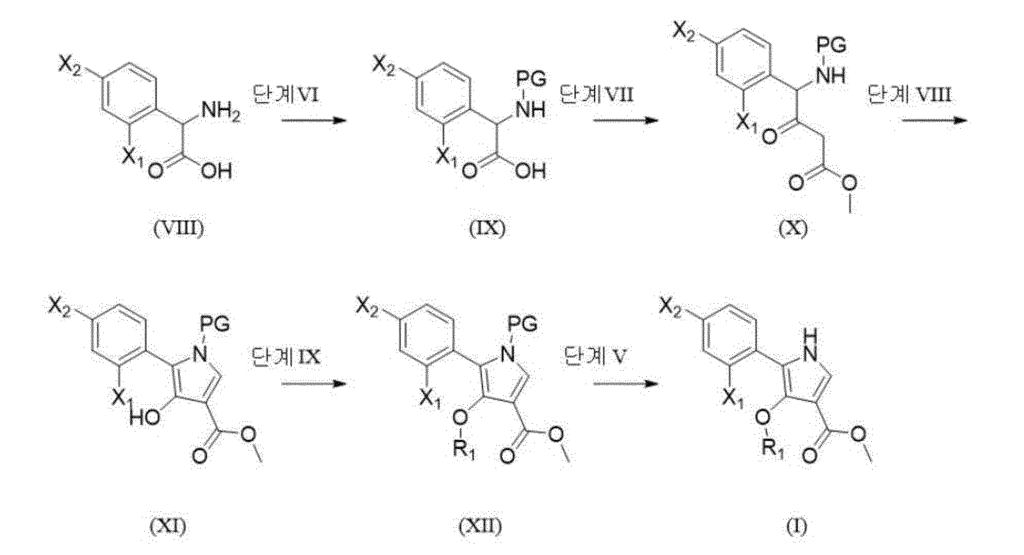
Synthesis Example 1. Synthesis of Example 1
[267]
[Example 1] 1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)- N -methylmethanamine
[268]
(1) Synthesis of step methyl 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrole-3-carboxylate
[269]Methyl 5-(2-fluorophenyl)-4-methoxy-1
H -pyrrole-3-carboxylate (intermediate 1, 1.0 eq., 1.2 g, 4.8 mmol) was dissolved in THF (20.0 mL), and NaH (2.0 eq., 384.8 mg, 9.6 mmol) was added dropwise at 0 °C and stirred at room temperature for 10 min. 6-Methoxypyridine-3-sulfonyl chloride (1.5 eq., 1.6 g, 7.2 mmol) was added and stirred at room temperature for 1 h. Water was added to the reaction solution, and the mixture was extracted with EA. The organic layer was dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by column chromatography to obtain methyl 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carboxylate as a light brown solid. (1.85 g, 91.6%)
[270]
(2) Synthesis of step 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)methanol
[271]Methyl 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carboxylate (1.0 eq., 1.0 g, 2.38 mmol) was dissolved in THF (5.0 mL), and 1.0 M DIBAL in
n -hexane solution (5.0 eq., 11.9 mL, 11.9 mmol) was added dropwise at 0 °C, followed by stirring at room temperature for 1 h. The reaction solution was cooled to 0 °C, quenched with an aqueous Rochelle salt solution, and extracted with EA. The organic layer was dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by column chromatography to obtain 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrol-3-yl)methanol as a yellow oil. (654.8 mg, 70.2%)
[272]
(3) Synthesis of step 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrole-3-carbaldehyde
[273]5-(2-Fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrol-3-yl)methanol (1.0 eq., 500.0 mg, 1.3 mmol) and Dess-Martin periodinane (1.0 eq., 540.4 mg, 1.3 mmol) were dissolved in DCM (10.0 mL) and stirred at room temperature for 1 h. The reaction mixture was concentrated and purified by column chromatography to give 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carbaldehyde as a pale brown solid. (388.2 mg, 78.1%)
[274]
(4) Step 1 Synthesis of (5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)- N -methylmethanamine
[275]5-(2-Fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carbaldehyde (1.0 eq., 385.0 mg, 0.99 mmol) was dissolved in THF (5.0 mL), and 2.0 M methylamine in THF (10 eq., 4.9 mL, 9.9 mmol) was added. After stirring at room temperature for 1 h, the reaction mixture was cooled to 0 °C, and NaBH
4 (10 eq., 373.4 mg, 9.9 mmol) was added, followed by stirring at room temperature for 1 h. 6.0
N aqueous hydrogen chloride solution was slowly added dropwise to the reaction solution, and the resulting solid was filtered. The filtered solid was dissolved in water, 1
N aqueous sodium hydroxide solution was added, and extraction was performed with EA. The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)-
N -methylmethanamine as a white solid. (125.8 mg, 28.3%) [M+H] + : 405
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023113474&_cid=P22-MI1405-08231-1
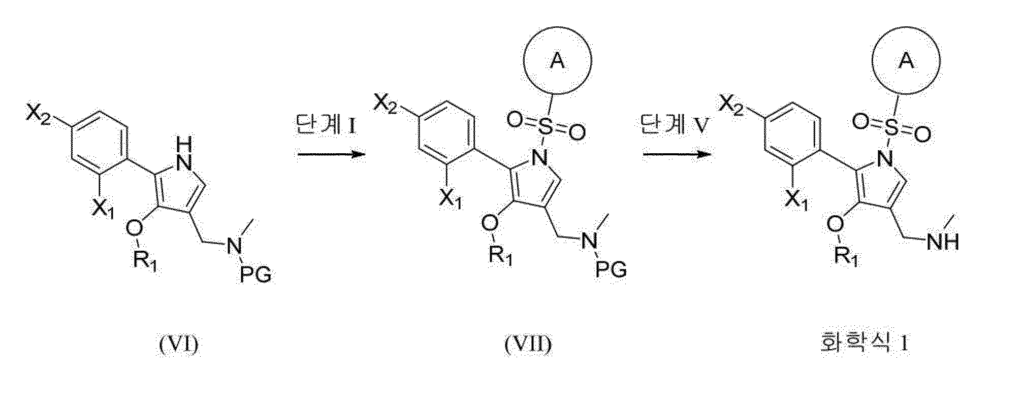
7) Preparation of 1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine free base[211]
(1) Step: Synthesis of methyl 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrole-3-carboxylate[212]Methyl 5-(2-fluorophenyl)-4-methoxy-1
H -pyrrole-3-carboxylate (intermediate 1, 1.0 eq., 920 g, 3.69 mol) was dissolved in DMF (9.2 L), and t-BuOK (2.0 eq., 828 g, 7.38 mmol) was added dropwise at 0 °C and stirred for 30 min. 6-Methoxypyridine-3-sulfonyl chloride (1.5 eq., 1.15 kg, 5.54 mol) was added and stirred at 0 °C for 1 h. Water was added to the reaction solution, which was then extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by column chromatography to obtain methyl 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carboxylate as a white solid. (1.20 kg, 77.4%) [213]
(2) Step: Synthesis of 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)methanol[214]Methyl 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carboxylate (1.0 eq., 1.1 kg, 2.62 mol) was dissolved in THF (11.0 L), and DIBAL 2.0 M in THF solution (3.0 eq., 3.93 L, 7.86 mol) was added dropwise at 0 °C, followed by stirring for 30 min. The reaction solution was quenched with 5% aqueous Rochelle’s salt solution and extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrol-3-yl)methanol as a light yellow oil. (870 g, 84.8%) [215]
(3) Step: Synthesis of 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrole-3-carbaldehyde[216]5-(2-Fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrol-3-yl)methanol (1.0 eq., 830 g, 2.12 mol) and TEA (4.0 eq., 1.59 kg, 15.7 mol) were dissolved in DMSO (4.15 L), and SO
3 -pyridine (4.0 eq., 1.35 kg, 8.48 mol) was added dropwise, and the mixture was stirred at room temperature for 1.5 h. Water was added to the reaction mixture at 0 °C, and the mixture was extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain 5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carbaldehyde as a yellow solid. (722 g, 87.6%) [217]
(4) Step: Synthesis of 1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)- N -methylmethanamine[218]5-(2-Fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1
H -pyrrole-3-carbaldehyde (1.0 eq., 715 g, 1.83 mol) was dissolved in methanol (7.2 L), and methylamine in methanol (5.0 eq., 916 g, 9.16 mol) was added. After stirring at room temperature for 1 h, the reaction mixture was concentrated, dissolved in ethanol (7.2 L), cooled to 0 °C, and NaBH
4 (2.0 eq., 139 g, 3.66 mol) was added, and stirred at 0 °C for 1 h. Water was added to the reaction solution, and extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by column chromatography to obtain 1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1 H -pyrrol-3-yl)-
N -methylmethanamine as a brown oil. (347 g, 46.7%)
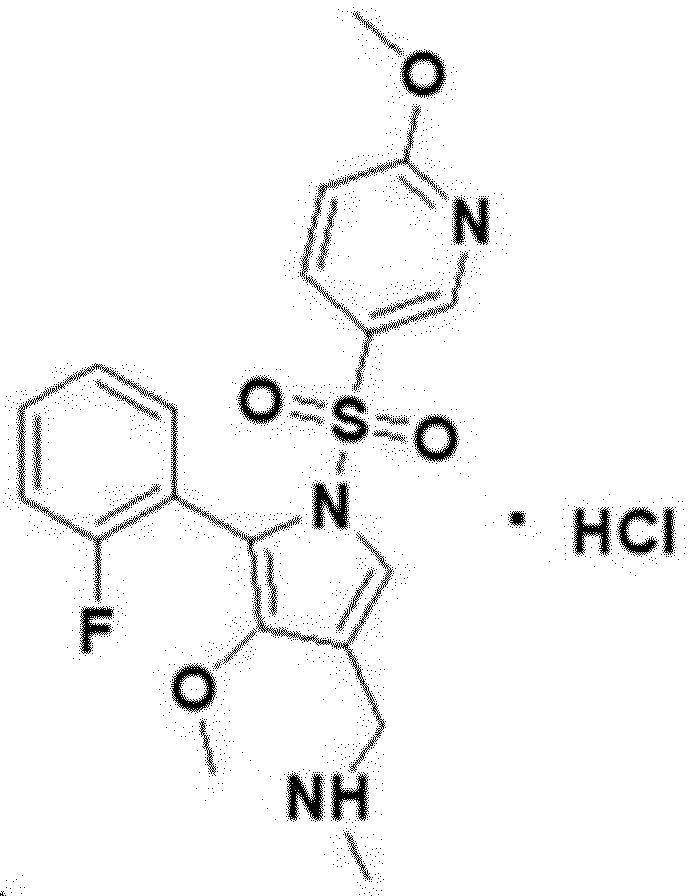
<Example 1> Preparation of hydrochloric acid salt of 1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine About 500 mg of the free base of 1-(5-(2-fluorophenyl)-4-methoxy-1-((6-methoxypyridin-3-yl)sulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine was weighed and placed in a glass vial, and then dissolved in 2 mL of ethanol while heating at 25°C. Then, 647.44 μL (2 M) hydrochloric acid was added to the vial. The sample was continuously stirred on a magnetic stirrer at room temperature for 24 hours, and after stirring for 24 hours, the solid precipitate was separated by centrifugation. Subsequently, the wet solid was dried at 40°C for 20 hours to obtain a grayish white dried powder.
SYN


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
/////////Padoprazan, proton pump inhibitor, 95BJ28E2RP, ID-120040002, ID 120040002














