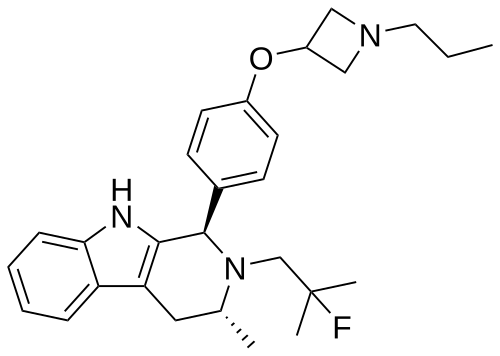
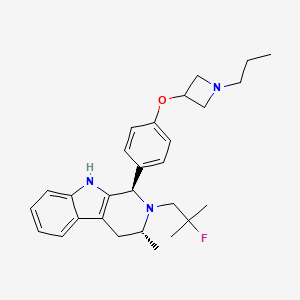
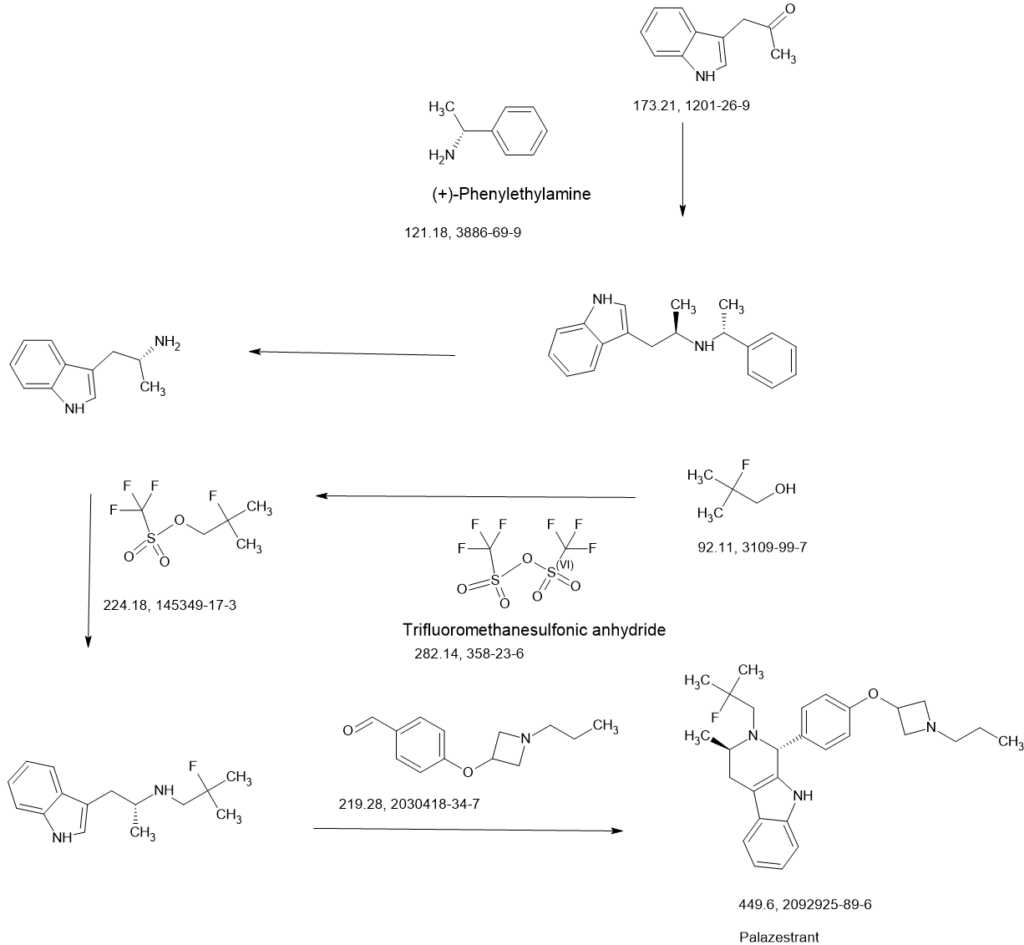
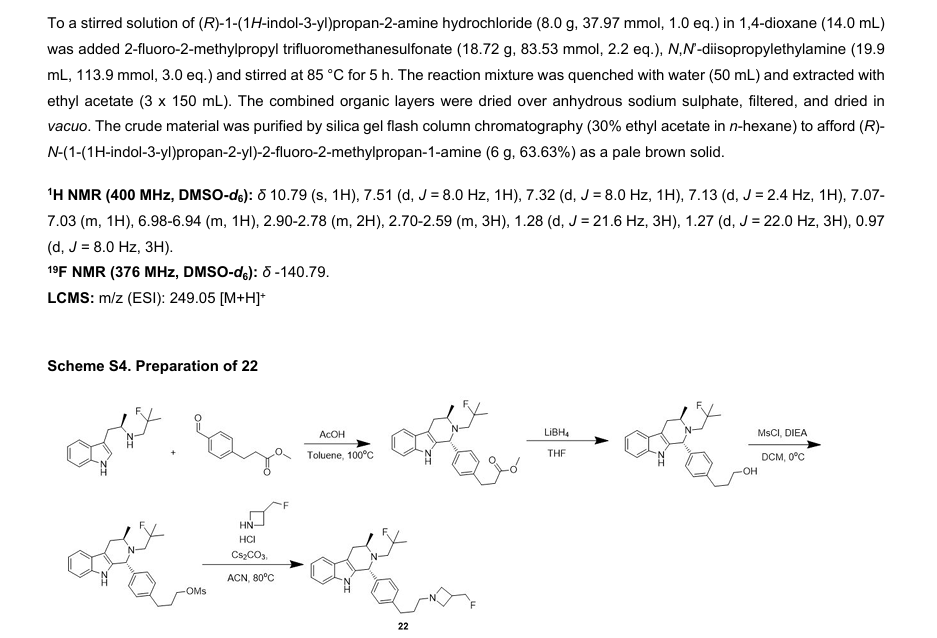
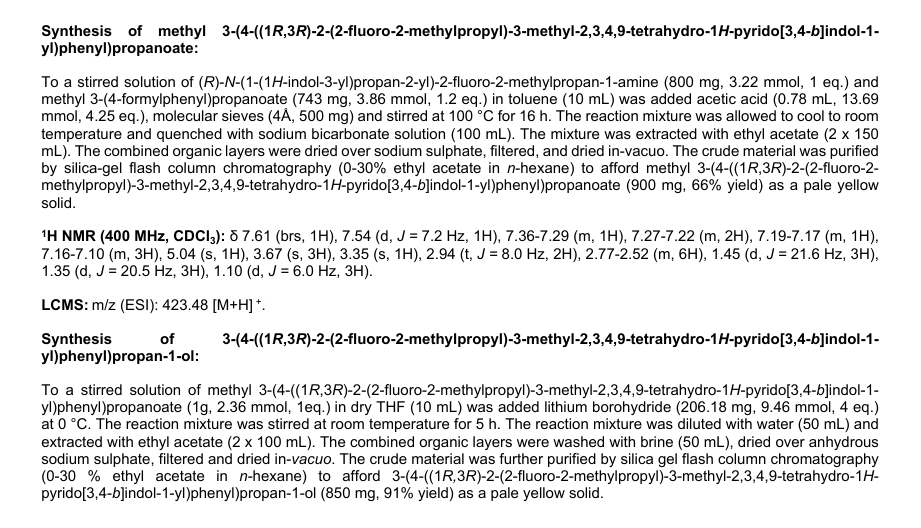
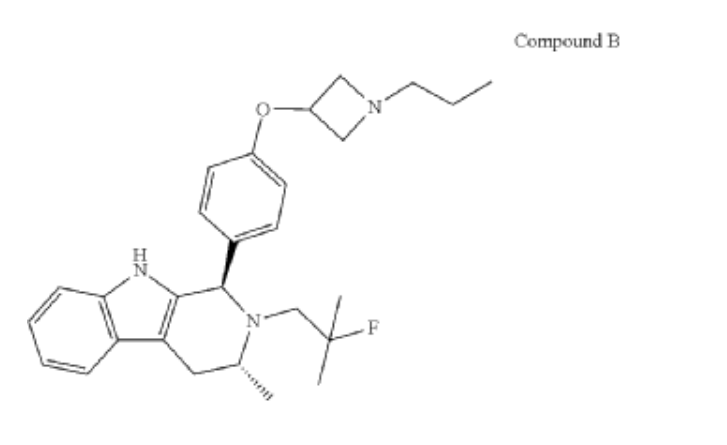
PALAZESTRANT
CAS 2092925-89-6
OP-1250, VU35KM56Q4
449.6 g/mol, C28H36FN3O
(1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-1-[4-(1-propylazetidin-3-yl)oxyphenyl]-1,3,4,9-tetrahydropyrido[3,4-b]indole
- (1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-1-[4-(1-propylazetidin-3-yl)oxyphenyl]-1,3,4,9-tetrahydropyrido[3,4-b]indole
- (1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-1-{4-[(1-propylazetidin-3- yl)oxy]phenyl}-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole
Palazestrant (OP-1250) is an investigational drug being developed for estrogen receptor-positive (ER+) breast cancer. It is a small molecule with a dual mechanism of action, acting as both a complete estrogen receptor antagonist and a selective estrogen receptor degrader (SERD). This means it can block estrogen receptor activity and also degrade the receptor itself, potentially offering a more effective treatment approach.
Here’s a more detailed breakdown:
- Dual Mechanism:Palazestrant is a complete ER antagonist, meaning it blocks all estrogen receptor activity. It is also a SERD, which means it degrades the estrogen receptor, preventing it from functioning.
- Oral Administration:Palazestrant is an orally available drug.
- Clinical Trials:Palazestrant is currently in clinical trials, including Phase 1/2 and Phase 3 studies, for the treatment of ER+, HER2- metastatic breast cancer.
- Combination Therapy:Palazestrant is being evaluated in combination with other drugs like CDK4/6 inhibitors (e.g., ribociclib).
- Promising Results:Preliminary results from clinical trials have shown promising antitumor efficacy and favorable pharmacokinetic properties for palazestrant.
- FDA Fast Track Designation:The FDA has granted Fast Track designation for the treatment of ER+/HER2- metastatic breast cancer that has progressed following endocrine therapy with a CDK4/6 inhibitor.
- Brain Metastasis:Palazestrant has shown activity in brain metastasis animal models.
- ESR1 Mutation Status:Palazestrant has demonstrated activity against both wild-type and mutant ER (ESR1) breast cancer models.
Palazestrant is an investigational new drug which is being evaluated for the treatment of estrogen receptor-positive (ER+) breast cancer, with a dual mechanism of action as both a complete estrogen receptor antagonist (CERAN) and a selective estrogen receptor degrader (SERD). This orally bioavailable small molecule has demonstrated potent activity against both wild-type and mutant forms of the estrogen receptor.[1]
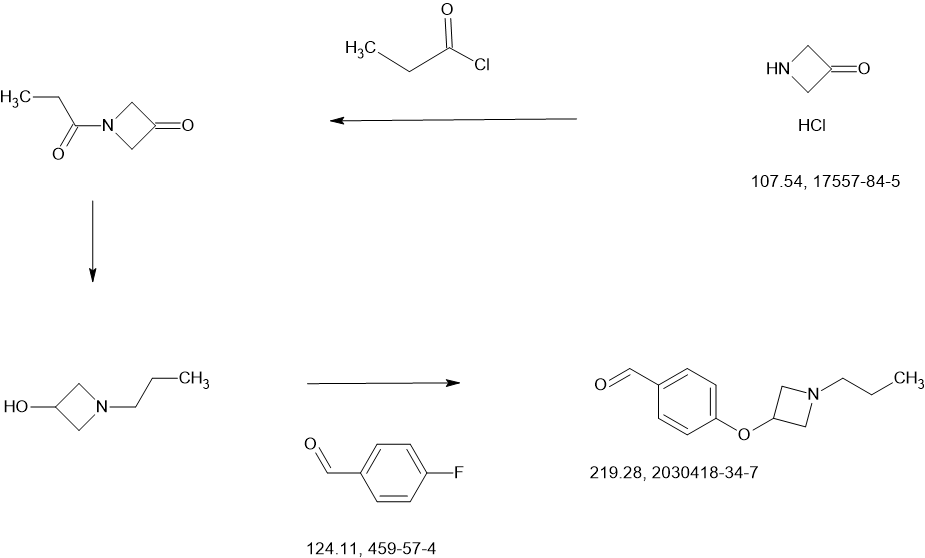
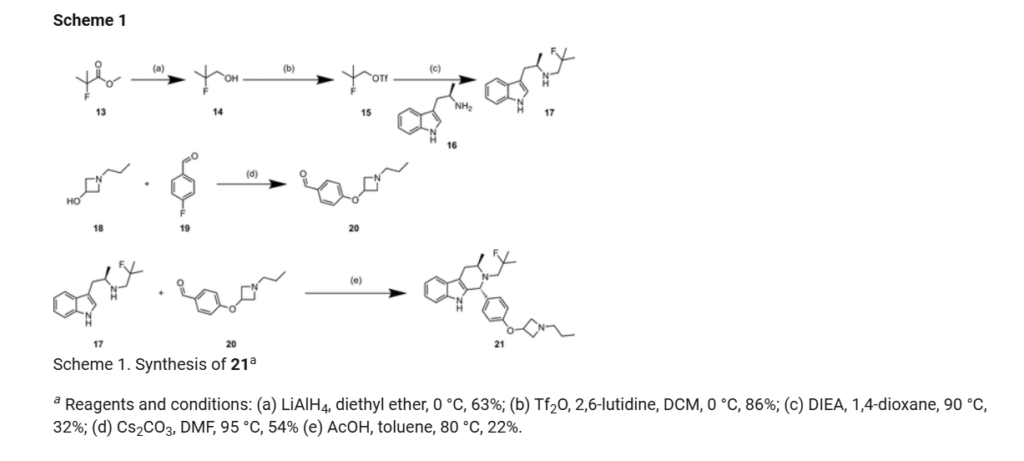
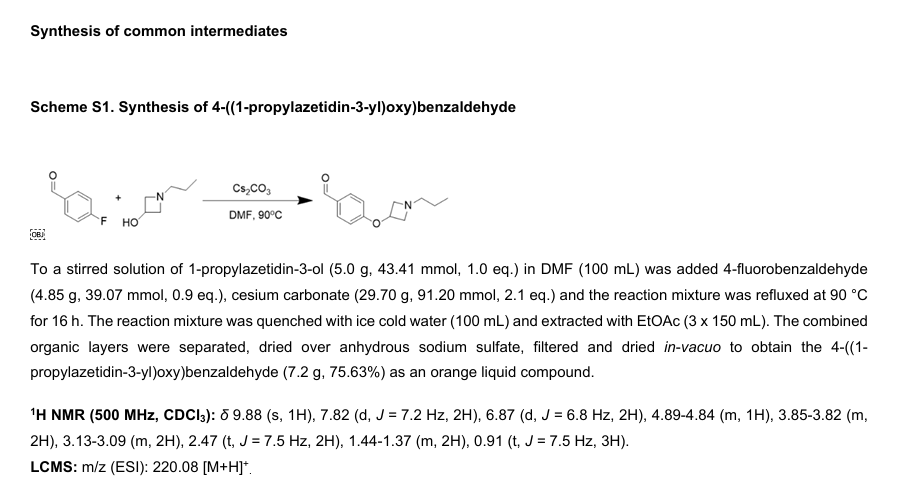
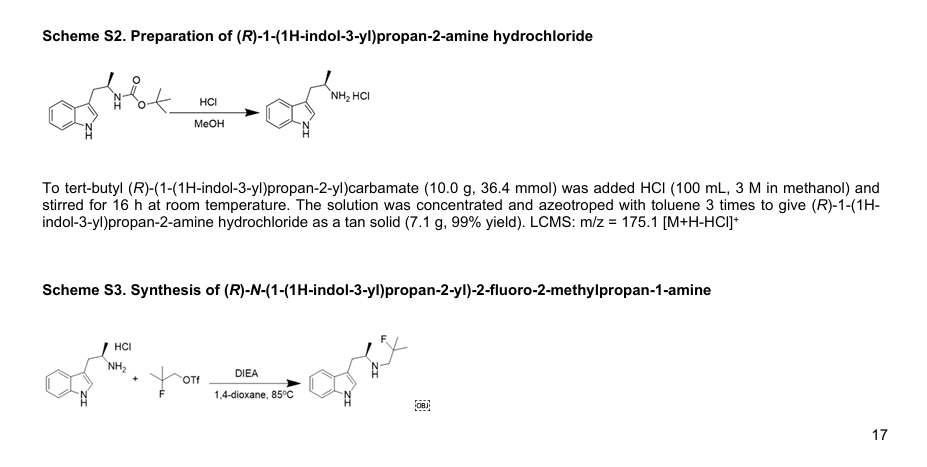
SCHEME
MAIN
PAPER
https://pubs.acs.org/doi/10.1021/acsomega.4c11023

PATENTS
https://patentscope.wipo.int/search/en/detail.jsf?docId=US379744130&_cid=P22-MCPZ5L-11621-1
PATENTS’
WO2017059139
WO2023225354
WO2023091550
WO2023283329
WO2021178846
References
- ^ Parisian AD, Barratt SA, Hodges-Gallagher L, Ortega FE, Peña G, Sapugay J, et al. (March 2024). “Palazestrant (OP-1250), A Complete Estrogen Receptor Antagonist, Inhibits Wild-type and Mutant ER-positive Breast Cancer Models as Monotherapy and in Combination”. Molecular Cancer Therapeutics. 23 (3): 285–300. doi:10.1158/1535-7163.MCT-23-0351. PMC 10911704. PMID 38102750.
| Clinical data | |
|---|---|
| Other names | OP-1250 |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 2092925-89-6 |
| PubChem CID | 135351887 |
| DrugBank | DB18971 |
| ChemSpider | 128922074 |
| UNII | VU35KM56Q4 |
| KEGG | D12827 |
| ChEMBL | ChEMBL5314475 |
| Chemical and physical data | |
| Formula | C28H36FN3O |
| Molar mass | 449.614 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
///////////PALAZESTRANT, OP 1250, A1AEA, VU35KM56Q4














