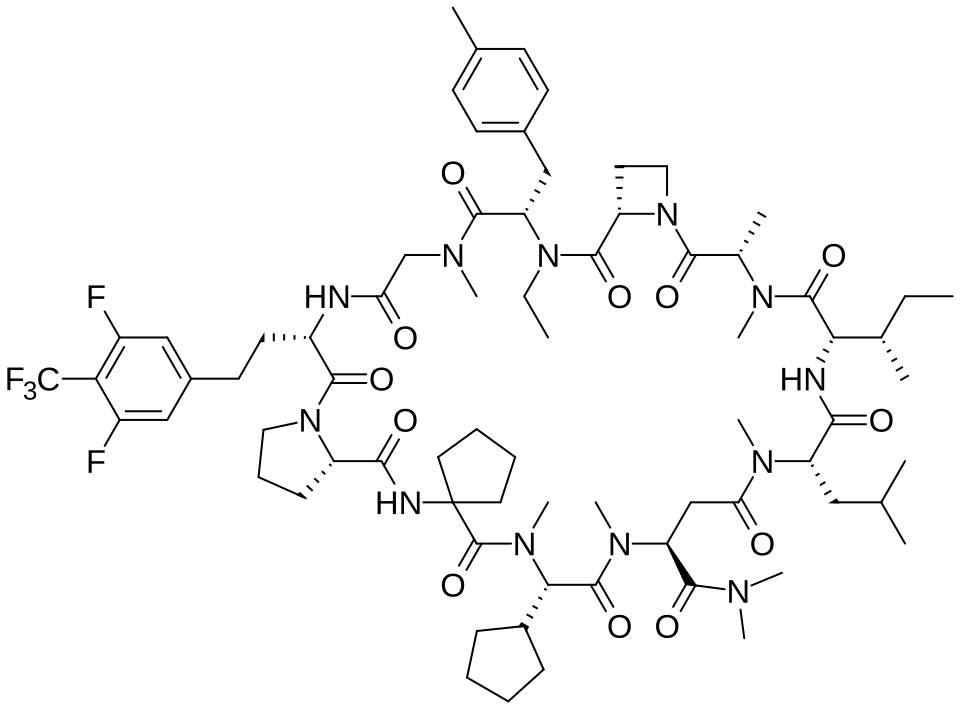


Paluratide
CAS 2676177-63-0
MFC73H105F5N12O12 MW 1437.7 g/mol
1,11-anhydro[N-methyl-L-alanyl-(2S)-azetidine-2-carbonyl-N-ethyl-4-methyl-L-phenylalanyl-N-methylglycyl-3-{[3,5-difluoro-4-(trifluoromethyl)phenyl]methyl}-L-alanyl-L-prolyl-2-
aminocyclopentane-1-carbonyl-(2S)-N-methyl-3-cyclopentylglycyl-1-
(dimethylamino)-N-methyl-L-aspart-4-yl-N-methyl-L-leucyl-Lisoleucine]
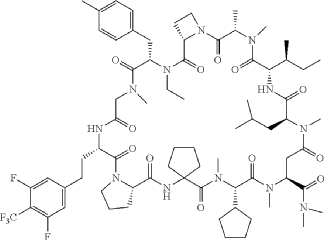
(3S,9S,12S,17S,20S,23S,27S,30S,36S)-20-[(2S)-butan-2-yl]-30-cyclopentyl-3-[2-[3,5-difluoro-4-(trifluoromethyl)phenyl]ethyl]-10-ethyl-N,N,7,17,18,24,28,31-octamethyl-9-[(4-methylphenyl)methyl]-23-(2-methylpropyl)-2,5,8,11,16,19,22,25,29,32,35-undecaoxospiro[1,4,7,10,15,18,21,24,28,31,34-undecazatricyclo[34.3.0.012,15]nonatriacontane-33,1′-cyclopentane]-27-carboxamide
G-protein Ras (rat sarcoma virus) inhibitor, antineoplastic, LUNA 18, CHUGAI, AW3YP3CD9X
Paluratide (development code LUNA18) was an investigational cyclic peptide KRAS inhibitor developed by Chugai Pharmaceutical, a member of the Roche Group, for the treatment of cancers with KRAS mutations.[1] The compound was notable as an orally bioavailable macrocyclic peptide that could target intracellular protein-protein interactions, a class of targets traditionally considered “undruggable.”[2]
Development was discontinued in July 2025 due to a narrow therapeutic window compared to competing KRAS inhibitors.[3]
Ras Inhibitor LUNA18 is an orally bioavailable cyclic peptide and Ras inhibitor, with potential antineoplastic activity. Upon oral administration, Ras inhibitor LUNA18 selectively targets, binds to and inhibits Ras, thereby inhibiting Ras-dependent signaling and inhibits proliferation of tumor cells in which Ras is overexpressed and/or mutated. Ras serves an important role in cell signaling, division and differentiation. Mutations of Ras may induce constitutive signal transduction leading to tumor cell growth, proliferation, invasion, and metastasis.
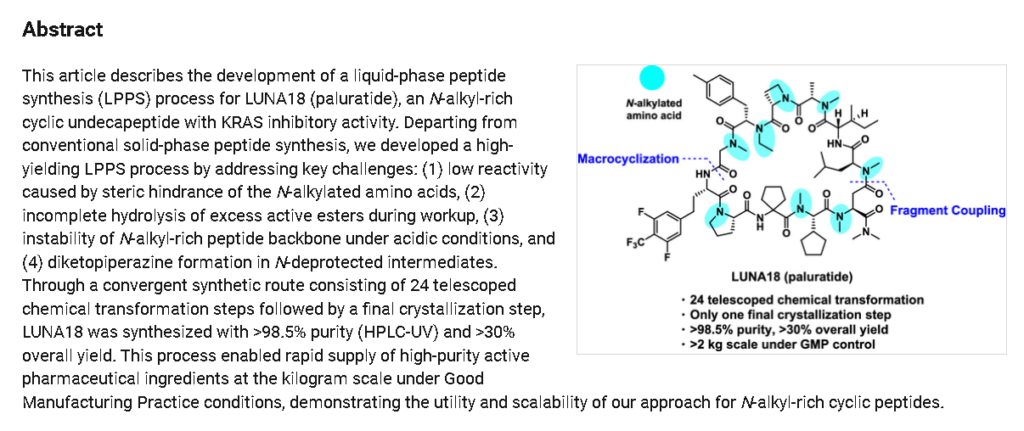
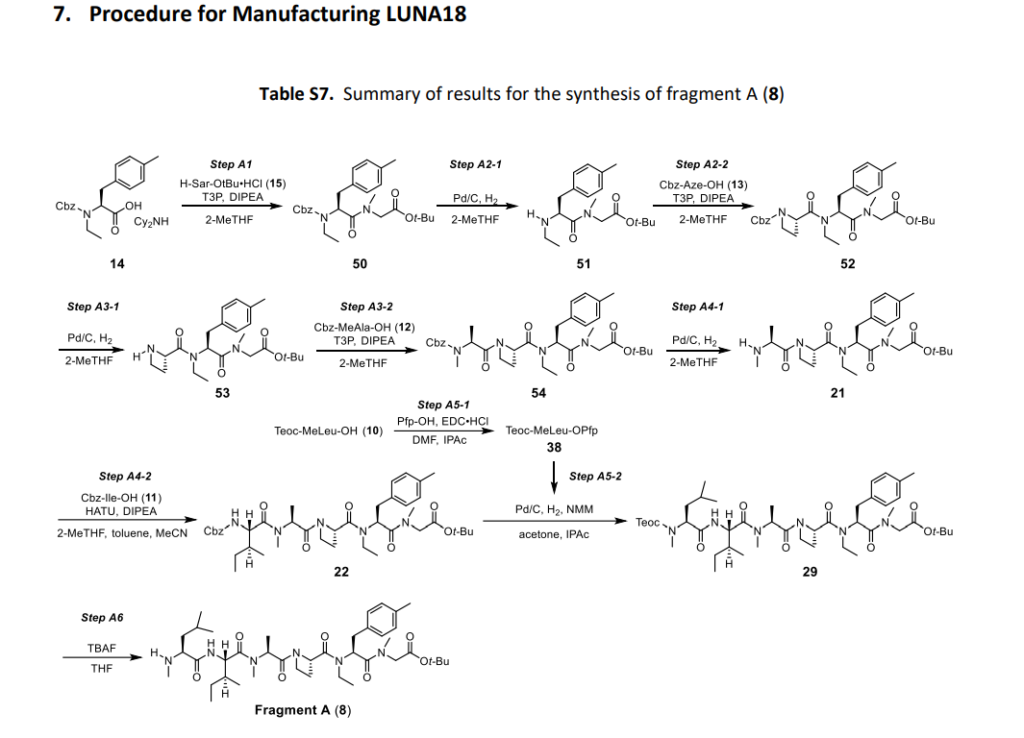
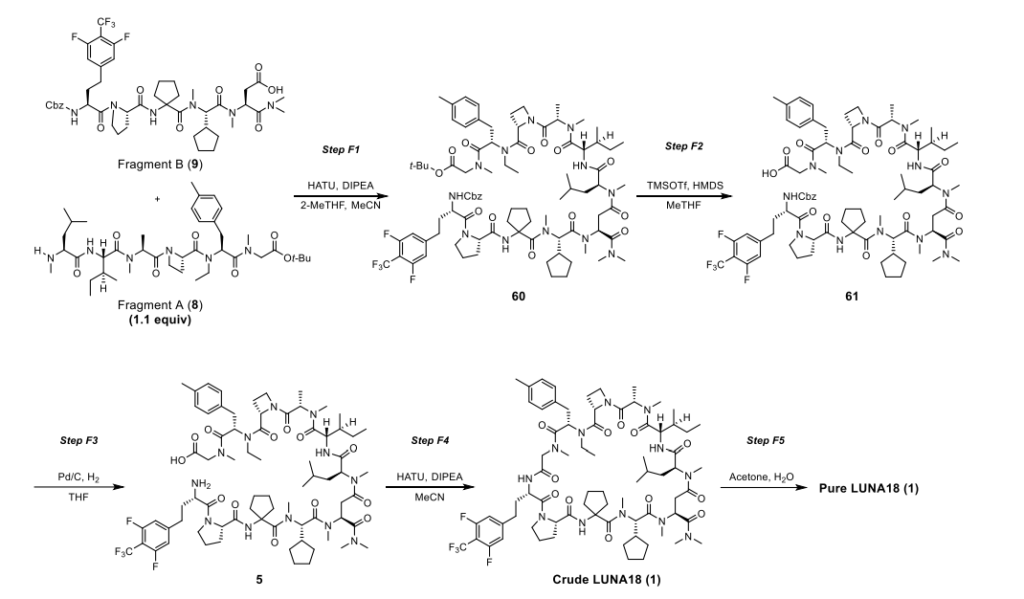
Paluratide (LUNA18 is synthesized using a novel liquid-phase peptide synthesis (LPPS) method, not traditional solid-phase methods, to overcome challenges with N-alkylated cyclic peptides. This process involves a convergent route of 24 telescoped chemical transformations, a final crystallization step, and a focus on specific strategies to manage side reactions like diketopiperazine formation and low reactivity of sterically hindered amino acids.
Key aspects of the synthesis
- Liquid-phase synthesis: A novel, high-yielding LPPS process was developed to enable the large-scale production of paluratide. This is a departure from traditional solid-phase methods, which have limitations with solubility and waste.
- Convergent synthetic route: The synthesis uses a convergent approach, meaning smaller fragments of the peptide are synthesized separately and then joined together. The overall process includes 24 telescoped chemical transformations followed by a final crystallization step.
- Addressing synthesis challenges: Specific strategies were employed to overcome key difficulties:
- Low reactivity: Amino acids with N-alkylation are sterically hindered, so more reactive and stable protecting groups were used to ensure efficient coupling.
- Side reactions: The method was designed to prevent side reactions like diketopiperazine formation in intermediates and incomplete hydrolysis of active esters.
- Instability: The peptide backbone is sensitive to acidic conditions, so a mildly acidic aqueous medium was chosen for workup and purification to maintain stability.
- Protecting group selection: Cbz-protected amino acid active esters were preferred over Boc-protected ones because they are less prone to forming N-carboxyanhydrides (NCA) under activating conditions, which can reduce yield and purity.
- Purification: A final crystallization step is used for purification.
PAT
- Method for producing eutectic of cyclic peptidePublication Number: WO-2024195801-A1Priority Date: 2023-03-20
- Method for producing cyclic peptide crystalsPublication Number: WO-2024085235-A1Priority Date: 2022-10-20
- Composition containing peptide, surfactant, and polymerPublication Number: WO-2024080308-A1Priority Date: 2022-10-12
- Methods for producing cyclic compounds comprising n-substituted amino acid residuesPublication Number: EP-4086272-A1Priority Date: 2021-05-07
- Methods for producing cyclic compounds comprising n-substituted amino acid residuesPublication Number: US-2022411462-A1Priority Date: 2021-05-07
SYN
https://pubs.acs.org/doi/10.1021/acs.oprd.5c00260?ref=PDF

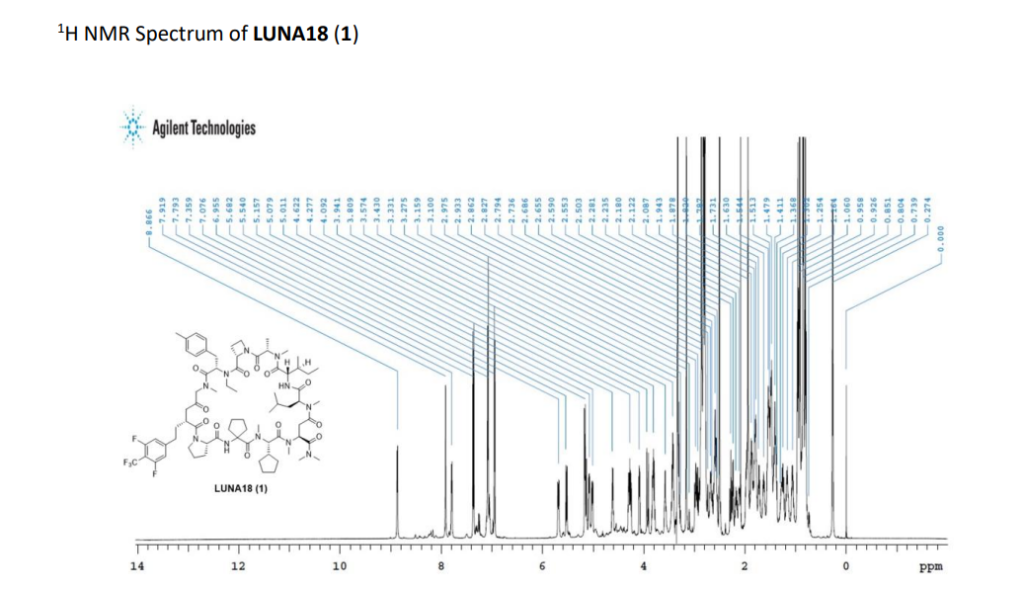
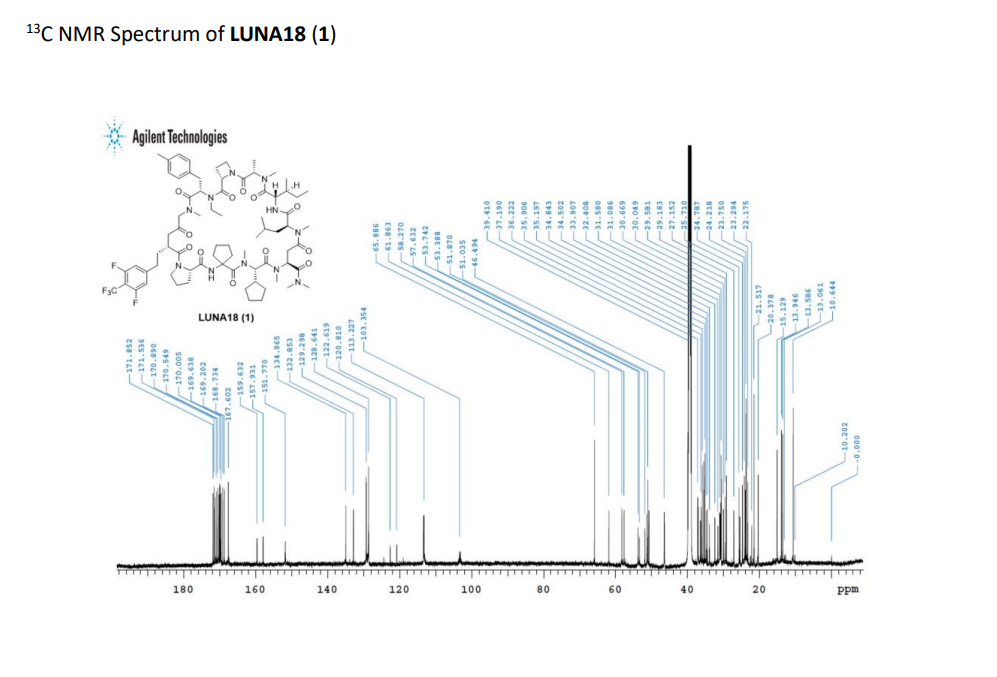
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US383248369&_cid=P20-MI3YXS-80609-1


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
Mechanism of action
Paluratide functions as a pan-RAS inhibitor, targeting multiple RAS isoforms including KRAS, NRAS, and HRAS.[1] The compound binds with high affinity to KRASG12D, with a dissociation constant (Kd) of 0.043 nM, and blocks the interaction between KRASG12D and the guanine nucleotide exchange factor SOS1 with an IC50 of less than 2.2 nM.[4]
Unlike covalent KRAS inhibitors that target specific mutations (such as sotorasib for KRASG12C), paluratide was designed to inhibit RAS proteins through disruption of protein-protein interactions with guanine nucleotide exchange factors (GEFs).[1] This mechanism allows the drug to affect RAS signalling regardless of the specific mutation, theoretically providing broader applicability across different KRAS-mutant cancers. The compound also demonstrates activity against downstream signalling pathways, affecting ERK and AKT phosphorylation.[4]
Medical uses
Paluratide was being developed for the treatment of locally advanced or metastatic solid tumors harbouring RAS gene alterations.[5] The drug demonstrated significant cellular activity against multiple cancer types with KRAS mutations in preclinical studies, including colorectal cancer, gastric cancer, non-small cell lung cancer, and pancreatic cancer.[1]
Chemistry
Paluratide is an 11-member (11-mer) cyclic peptide with a molecular weight in the range of 1000–2000 g/mol, classified as a “middle-size” cyclic peptide.[1] The compound features extensive N-alkylation, a modification that reduces hydrogen bond donors and improves oral absorption while maintaining cellular permeability.[2] Its structure allows it to navigate the challenging boundary between small molecules and biologics, achieving properties of both classes. The compound demonstrated oral bioavailability ranging from 21% to 47% in preclinical animal studies without requiring special formulations.[1]
Discovery
Paluratide was discovered through Chugai Pharmaceutical’s cyclic peptide platform using an mRNA display library screening approach.[1] The initial hit compound, designated AP8747, was identified from the mRNA display library and subsequently underwent extensive chemical optimization without scaffold hopping (maintaining the basic cyclic peptide structure).[1] The optimization focused on increasing plasma stability, improving absorption, reducing clearance, and reducing hydrogen bond donors to achieve oral bioavailability.
The final clinical compound, LUNA18, emerged after modifications to four amino acid positions (positions 5, 7, 10, and 11) from an intermediate compound (compound 40). Key structure-activity relationship findings included: the side chain at position 5 preferring aromatic over aliphatic groups; physicochemical properties being adjustable at position 11; and biological activity enhancement through modifications at positions 7 and 10.[1]
Chugai also developed a novel synthetic methodology that enabled the broadly applicable synthesis of highly N-alkylated cyclic peptide-like drugs.[6] This method overcame three major technical challenges: formation of diketopiperazine, insufficient reactivity of amidation due to steric hindrance, and instability of cyclic peptides under acidic conditions. Using this approach, more than 4,000 cyclic peptides were synthesized with a process yield of 31% and final product purity of 97%.[6]
Clinical trials
A Phase 1 dose-escalation and cohort expansion study (NCT05012618) was initiated in August 2021 to evaluate the safety, pharmacokinetics, pharmacodynamics, and preliminary activity of paluratide administered as a single agent or in combination with other anti-cancer drugs.[5] The study, in the United States and Japan, was designed to enrol approximately 195 patients with locally advanced or metastatic solid tumors positive for documented RAS alterations.[5]
Paluratide was administered orally as capsules.[5] The study also evaluated combination therapy with cetuximab, an EGFR inhibitor.[5]
References
- Tanada M, Tamiya M, Matsuo A, Chiyoda A, Takano K, Ito T, et al. (August 2023). “Development of Orally Bioavailable Peptides Targeting an Intracellular Protein: From a Hit to a Clinical KRAS Inhibitor”. Journal of the American Chemical Society. 145 (30): 16610–16620. Bibcode:2023JAChS.14516610T. doi:10.1021/jacs.3c03886. PMID 37463267.
- Ohta A, Tanada M, Shinohara S, Morita Y, Nakano K, Yamagishi Y, et al. (November 2023). “Validation of a New Methodology to Create Oral Drugs beyond the Rule of 5 for Intracellular Tough Targets”. Journal of the American Chemical Society. 145 (44): 24035–24051. Bibcode:2023JAChS.14524035O. doi:10.1021/jacs.3c07145. PMID 37874670.
- Taylor NP (24 October 2025). “Roche axes 4 Chugai solid tumor assets in early-phase clear-out”. Fierce Biotech.
- “LUNA18 (Paluratide) – KRAS Inhibitor, ERK Inhibitor, RAS Inhibitor”. MedChemExpress.
- “A Dose-escalation Study of LUNA18 in Patients With Locally Advanced or Metastatic Solid Tumors (With Expansion)”. ClinicalTrials.gov. 29 July 2025. NCT05012618.
- Nomura K, Hashimoto S, Takeyama R, Tamiya M, Kato T, Muraoka T, et al. (October 2022). “Broadly Applicable and Comprehensive Synthetic Method for N-Alkyl-Rich Drug-like Cyclic Peptides”. Journal of Medicinal Chemistry. 65 (19): 13401–13412. doi:10.1021/acs.jmedchem.2c01296. PMID 36109865.
- “Chugai Announces 2025 2nd Quarter Results” (Press release). Chugai Pharmaceutical. 24 July 2025.
External links
- Phase 1 Clinical Trial Information at ClinicalTrials.gov
- Development of LUNA18 at Journal of the American Chemical Society
| Clinical data | |
|---|---|
| Other names | LUNA18 |
| Routes of administration | Oral administration |
| Legal status | |
| Legal status | Development discontinued |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2676177-63-0 |
| PubChem CID | 166509683 |
| ChemSpider | 129321315 |
| UNII | AW3YP3CD9X |
| Chemical and physical data | |
| Formula | C73H105F5N12O12 |
| Molar mass | 1437.707 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
//////Paluratide, antineoplastic, LUNA 18, CHUGAI, AW3YP3CD9X














