
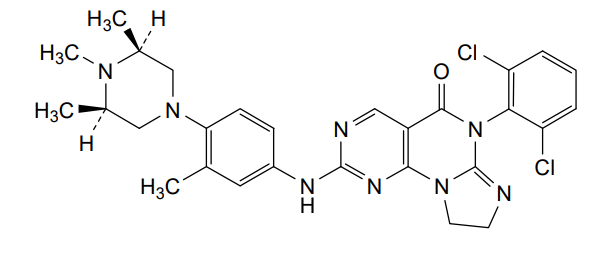
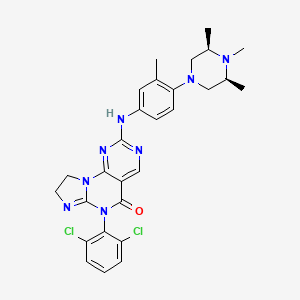
Potrasertib
CAS 2226938-19-6
MFC28H30Cl2N8O MW 565.5 g/mol
6-(2,6-dichlorophenyl)-2-{3-methyl-4-[(3R,5S)-3,4,5-trimethylpiperazin-1-yl]anilino}-8,9-dihydroimidazo[1,2-a]pyrimido[5,4-e]pyrimidin-5(6H)-one
7-(2,6-dichlorophenyl)-12-[3-methyl-4-[(3S,5R)-3,4,5-trimethylpiperazin-1-yl]anilino]-2,5,7,11,13-pentazatricyclo[7.4.0.02,6]trideca-1(13),5,9,11-tetraen-8-one
serine/ threonine kinase inhibitor, antineoplastic, IMP 7068, WEE1-IN-10, orb2664172, 621K13UG4B, Phase 1, Solid tumours
- OriginatorIMPACT Therapeutics
- ClassAntineoplastics; Small molecules
- Mechanism of ActionWEE1 protein inhibitors
- Phase ISolid tumours
- 28 Mar 2024No recent reports of development identified for phase-I development in Solid-tumours(Late-stage disease, Monotherapy) in Taiwan (PO)
- 28 Mar 2024No recent reports of development identified for phase-I development in Solid-tumours(Late-stage disease, Monotherapy) in USA (PO)
- 20 Oct 2023Efficacy, adverse events, pharmacodynamics and pharmacokinetics data from the phase I WEE1 trial in Solid tumours presented at the 48th European Society for Medical Oncology Congress (ESMO-2023)
Potrasertib is an investigational drug that is a selective inhibitor of WEE1 kinase, a protein crucial for the cell cycle. It is being studied for the treatment of various advanced solid tumors, including small cell lung cancer, ovarian, and colorectal cancers. By blocking the WEE1 kinase, potrasertib causes cancer cells with DNA damage to undergo premature, error-prone mitosis, which leads to cell death.
How it works
- Potrasertib is a serine/threonine kinase inhibitor.
- It works by targeting WEE1 kinase, which regulates the cell’s response to DNA damage.
- By inhibiting WEE1, it prevents cancer cells from repairing DNA damage before dividing, forcing them into a state that leads to cell death.
- This mechanism is particularly effective in tumors with a defective p53 gene, as these tumors rely more heavily on the WEE1 checkpoint for survival.
Potential uses
- Combination therapy: It is being explored in combination with chemotherapy (like gemcitabine and cisplatin) or radiotherapy to enhance their effectiveness against cancer.
- Monotherapy: It is also being studied as a standalone treatment for certain cancers, including ovarian, colorectal, and non-small cell lung cancer, especially those with high replication stress or WEE1 dependency.
Current status
- Potrasertib is still an investigational drug and is not yet approved for widespread clinical use.
- It is undergoing clinical trials to evaluate its safety and effectiveness in treating advanced cancers.
Potrasertib is an investigational new drug that is being evaluated by IMPACT Therapeutics for the treatment of advanced solid tumors. It is oral inhibitor of WEE1 kinase, a key regulator of cell cycle checkpoints.[1][2]
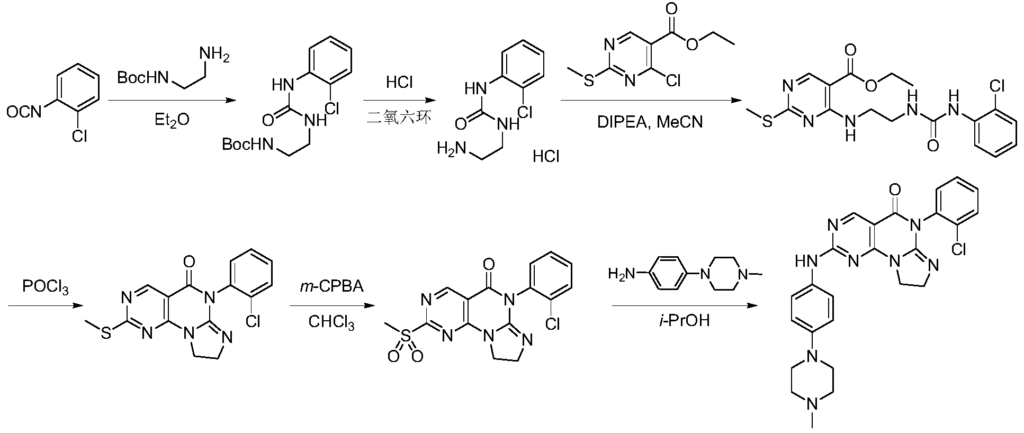
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018090939&_cid=P21-MI6TEY-70275-1
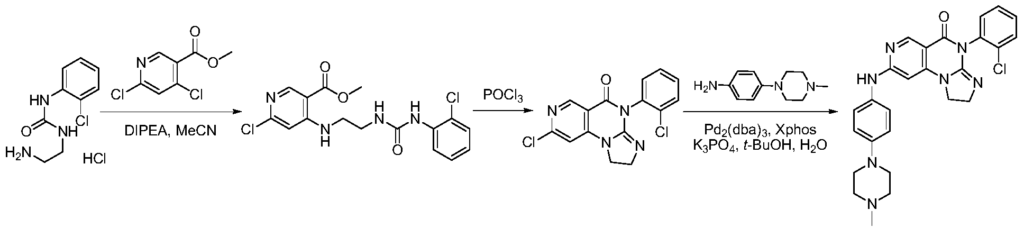
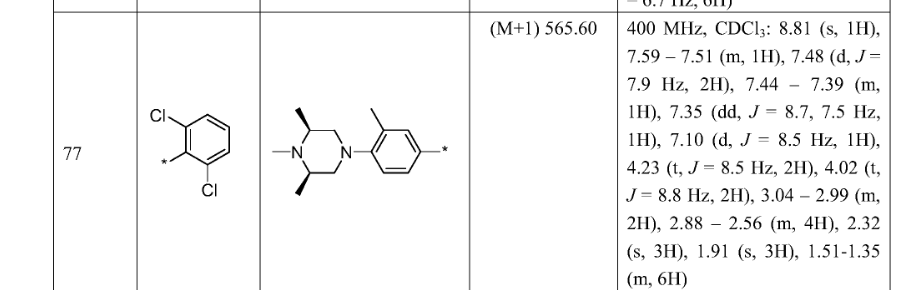
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021073491&_cid=P21-MI6TF3-70349-1
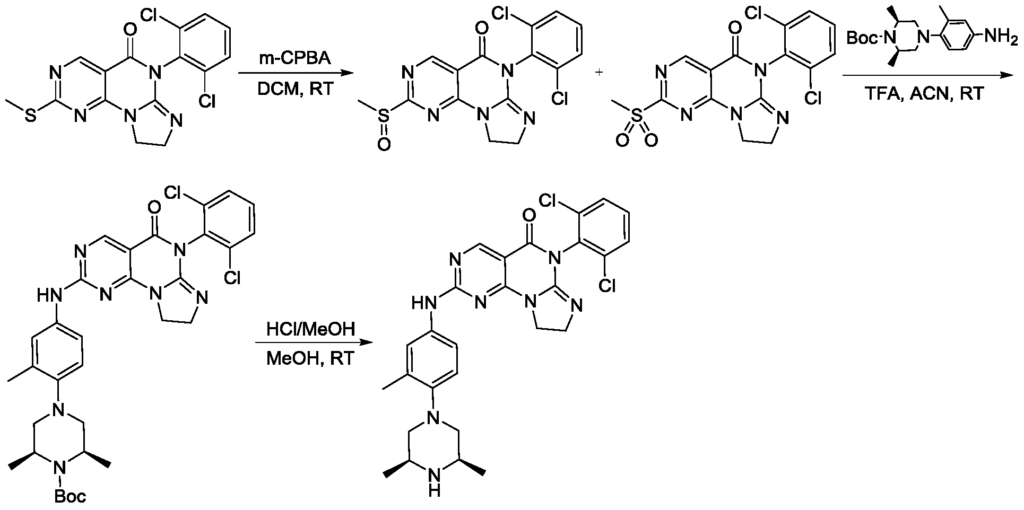
Example 1
SIMILAR NOT SAME
[0117]6-(2,6-dichlorophenyl)-2-((4-((3S,5R)-3,5-dimethylpiperazin-1-yl)-3-methylphenyl)amino)-8,9-dihydroimidazo[1,2-a]pyrimidino[5,4-e]pyrimidin-5(6H)-one
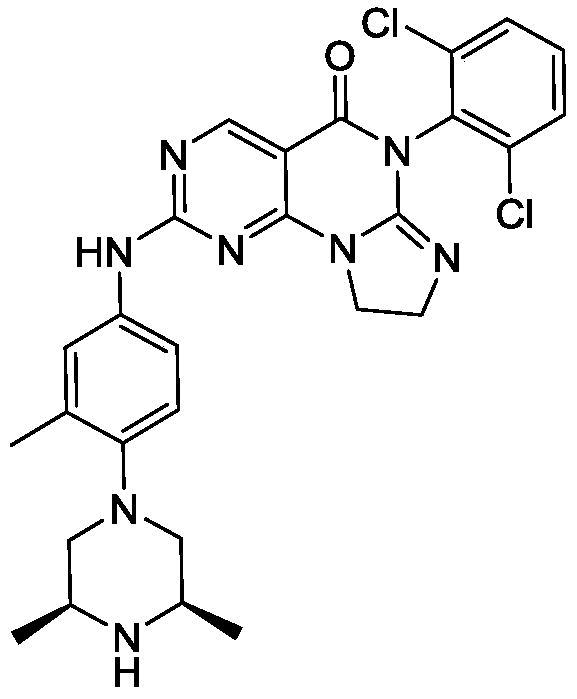
SIMILAR NOT SAME
[0128]6-(2,6-dichlorophenyl)-2-((4-((3S,5R)-3,5-dimethyl-4-(methyl-d3)piperazin-1-yl)-3-methylphenyl)amino)-8,9-dihydroimidazo[1,2-a]pyrimidino[5,4-e]pyrimidin-5(6H)-one
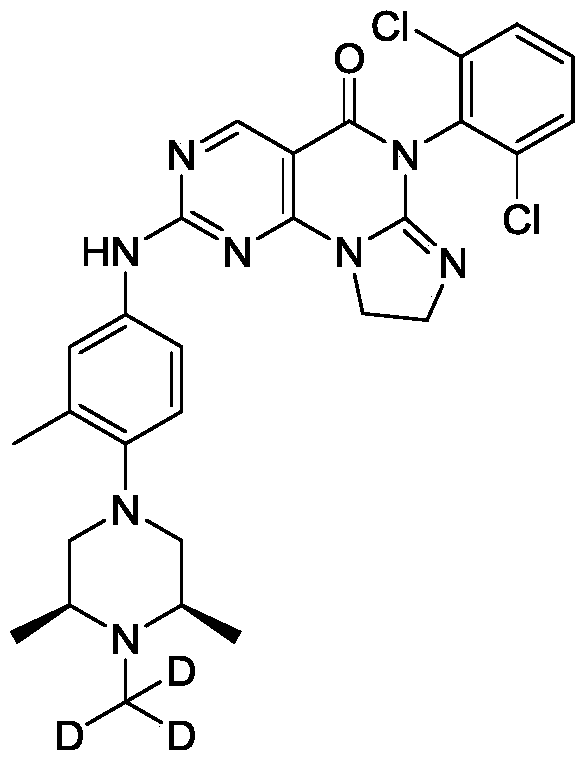
[0130]a) Preparation of (2S,6R)-2,6-dimethyl-1-(methyl-d3)-4-(2-methyl-4-nitro)piperazine: Sodium hydride (385.03 mg, 9.63 mmol, 60% purity) was added to a solution of (3S,5R)-3,5-dimethyl-1-(2-methyl-4-nitro)piperazine (2 g, 8.02 mmol) in N,N-dimethylformamide (15 mL). The mixture was stirred at 0 °C for 25 hours, then trideuterated iodomethane (1.16 g, 8.02 mmol, 499.09 μL) was added, and the mixture was stirred at 0 °C for 2 hours. The reaction was quenched by adding an aqueous sodium bicarbonate solution (30 mL) at 0 °C, extracted with ethyl acetate (50 mL × 3), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain the target crude product (1.5 g, yellow-green solid). LC-MS(ESI): m/z(M+1) + 267.1. 1 H NMR (400MHz, CDCl
3 ): δ8.04-8.01 (m, 2H), 6.96 (d, J = 12.0Hz, 1H), 3.10 (d, J = 12Hz, 2H), 2.65 (t , J=12Hz, 2H), 2.45-2.43 (m, 2H), 2.36 (s, 3H), 1.16-1.15 (d, J=4.0Hz, 6H).
[0131]b) Preparation of 4-((3S,5R)-3,5-dimethyl-4-(methyl-d3)piperazin-1-yl)-3-methylaniline: Under nitrogen protection, palladium on carbon (281.58 μmol, 10% purity) was added to a methanol (5 mL) solution of (2S,6R)-2,6-dimethyl-1-(methyl-d3)-4-(2-methyl-4-nitro)piperazine (1.5 g, 5.63 mmol). The resulting suspension was purified multiple times under vacuum with hydrogen. The mixture was stirred at 25 °C for 12 hours under a hydrogen atmosphere (15 psi). The reaction mixture was filtered, and the filtrate was concentrated under reduced pressure to give the target crude product (1.3 g, black solid). LC-MS (ESI): m/z (M+1) + 237.1.
[0132]c) Preparation of 6-(2,6-dichlorophenyl)-2-((4-(((3S,5R)-3,5-dimethyl-4-(methyl-d3)piperazin-1-yl)-3-methylphenyl)amino)-8,9-dihydroimidazo[1,2-a]pyrimidino[5,4-e]pyrimidin-5(6H)-one: 4-((3S,5R)-3,5-dimethyl-4-(methyl-d3)piperazin-1-yl)-3-methylaniline (459.32 mg, 1.94 mmol) and the prepared 6-(2,6-dichlorophenyl)-2- A mixture (700 mg, crude) of crude (methanesulfonyl)-8,9-dihydroimidazo[1,2-a]pyrimido[5,4-e]pyrimidin-5(6H)-one and 6-(2,6-dichlorophenyl)-2-(methanesulfonyl)-8,9-dihydroimidazo[1,2-a]pyrimido[5,4-e]pyrimidin-5(6H)-one was dissolved in acetonitrile (5 mL) and trifluoroacetic acid (20.14 mg, 0.177 mmol, 13.08 μL) was added. The mixture was stirred at 20–25 °C for 2 hours, filtered, and the filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by reversed-phase HPLC to give the target compound (56.89 mg, 100.00 μmol, yellow solid, 5.66% yield). LC-MS (ESI): m/z (M+1) + 568.0.
1 H NMR (400MHz, CDCl 3 ): δ8.81 (s, 1H), 7.49 (d, J=3.8Hz, 3H), 7.41-7.34 (m, 3H), 7.02 (d, J=4.2Hz, 1H), 4.25-4.21 (m, 2H), 4.02 (t, J=8.0Hz, 2H), 2.95 (d, J=6.0Hz 2H), 2.62 (t, J=6.0Hz, 2H), 2.46-2.41 (m, 2H), 2.34 (s, 6H), 1.15 (d, J=6.4Hz, 6H).
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022188802&_cid=P21-MI6TVM-79837-1
PAT
- 8,9-dihydroimidazo[1,2-a]pyrimido[5,4-e]pyrimidin-5(6H)-onesPublication Number: US-11345711-B2Priority Date: 2016-11-16Grant Date: 2022-05-31
- 8,9-dihydroimidazole[1,2-a]pyrimido[5,4-e]pyrimidine-5(6h)-ketone compoundPublication Number: EP-3543242-B1Priority Date: 2016-11-16Grant Date: 2024-01-03
- Compound 8,9-dihydroimidazole[1,2-a]pyrimido[5,4-e]pyrimidin-5(6H)-ketonePublication Number: ES-2968252-T3Priority Date: 2016-11-16Grant Date: 2024-05-08
- 8,9-dihydroimidazole[1,2-a]pyrimido[5,4-e]pyrimidine-5(6h)-ketone compoundPublication Number: EP-3543242-A1Priority Date: 2016-11-16
- 8,9-dihydroimidazo[1,2-a]pyrimido[5,4-e]pyrimidin-5(6H)-onesPublication Number: US-10703759-B2Priority Date: 2016-11-16Grant Date: 2020-07-07
- 8,9-DIHYDROIMIDAZO[1,2-a]PYRIMIDO[5,4-e]PYRIMIDIN-5(6H)-ONESPublication Number: US-2019308984-A1Priority Date: 2016-11-16
- 8,9-DIHYDROIMIDAZO[1,2-a]PYRIMIDO[5,4-e]PYRIMIDIN-5(6H)-ONESPublication Number: US-2020385394-A1Priority Date: 2016-11-16
- 8,9-Dihydroimidazo[1,2-a]pyrimido[5,4-e]pyrimidin-5(6H)-onesPublication Number: CN-109906227-BPriority Date: 2016-11-16Grant Date: 2022-03-11
- Dihydroimidazo pyrimido pyrimidinone compoundPublication Number: WO-2021073491-A1Priority Date: 2019-10-16
- DihydroimidazopyrimidopyrimidinonesPublication Number: CN-114502559-APriority Date: 2019-10-16
- Dihydroimidazopyrimidopyrimidinone compoundsPublication Number: CN-114502559-BPriority Date: 2019-10-16Grant Date: 2024-02-02
- Dihydroimidazo pyrimido pyrimidinone compoundPublication Number: US-2024010655-A1Priority Date: 2019-10-16
- 8,9-dihydroimidazo[1,2-a]pyrimido[5,4-e]pyrimidin-5(6h)-onesPublication Number: CA-3043945-A1Priority Date: 2016-11-16
- Use of Wee1 kinase inhibitors in the treatment of cancerPublication Number: CN-118338905-APriority Date: 2021-11-26
- Use of wee1 kinase inhibitors in the treatment of cancerPublication Number: WO-2023093840-A1Priority Date: 2021-11-26
- Use of wee1 kinase inhibitors in the treatment of cancerPublication Number: WO-2022188802-A1Priority Date: 2021-03-10
- The use of Wee1 kinase inhibitors in the treatment of cancer diseasesPublication Number: CN-117202908-APriority Date: 2021-03-10
- Use of wee1 kinase inhibitors in the treatment of cancerPublication Number: US-2024091233-A1Priority Date: 2021-03-10



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | IMP7068 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2226938-19-6 |
| PubChem CID | 139503236 |
| UNII | 621K13UG4B |
| Chemical and physical data | |
| Formula | C28H30Cl2N8O |
| Molar mass | 565.50 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- “IMP 7068”. AdisInsight. Springer Nature Switzerland AG.
- Wang Z, Li W, Li F, Xiao R (January 2024). “An update of predictive biomarkers related to WEE1 inhibition in cancer therapy”. Journal of Cancer Research and Clinical Oncology. 150 (1): 13. doi:10.1007/s00432-023-05527-y. PMC 10794259. PMID 38231277.
///////potrasertib, antineoplastic, IMP 7068, WEE1-IN-10, orb2664172, 621K13UG4B, Phase 1, Solid tumours














