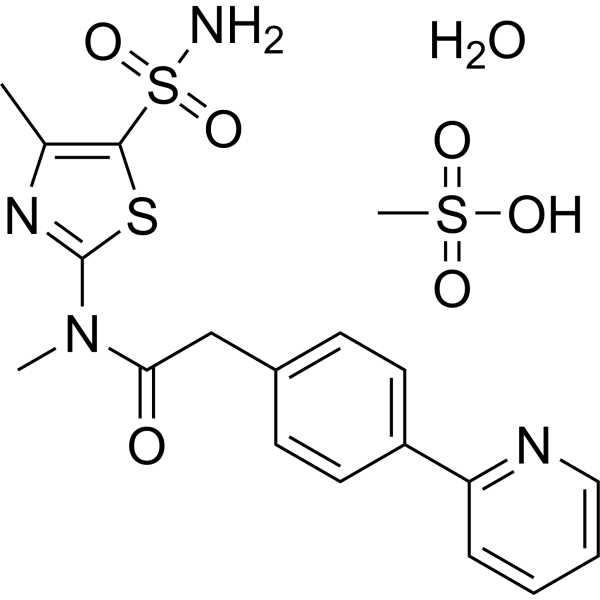
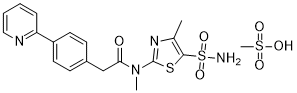
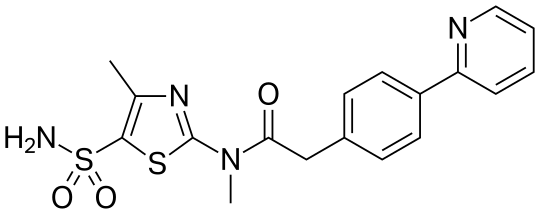
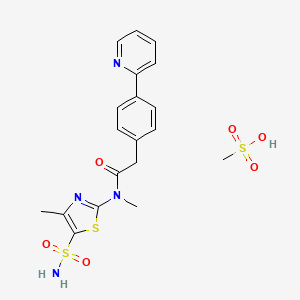
PRITELIVIR MESYLATE
CAS 1428333-96-3
1428321-10-1 HYDRATE
FREE FORM
AIC316 mesylate hydrate; BAY 57-1293 mesylate hydrate
BAY57-1293; BAY 57-1293; BAY-57-1293; BAY571293; BAY 571293; BAY-571293; AIC-316; AIC 316
| Molecular Weight | 516.61 |
|---|---|
| Synonyms | AIC316 mesylate hydrate; BAY 57-1293 mesylate hydrate |
| Formula | C19H24N4O7S3 |
| CAS No. | 1428321-10-1 |
Pritelivir mesylate is an antiviral drug currently under development, specifically targeting herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). It functions by inhibiting the viral helicase-primase enzyme, a crucial component for HSV replication. It is being investigated as a potential treatment for various herpes infections, including those resistant to traditional antivirals like acyclovir.
Key aspects of Pritelivir mesylate:
- Mechanism of Action:Pritelivir is a helicase-primase inhibitor, meaning it blocks the activity of an enzyme essential for the replication of herpes viruses.
- Target Viruses:It is effective against both HSV-1 and HSV-2, the viruses responsible for cold sores and genital herpes, respectively.
- Potential for Resistance:Pritelivir has shown promise in preclinical studies against acyclovir-resistant strains of HSV, making it a potential alternative for patients with drug-resistant infections.
- Clinical Trials:Pritelivir is currently in phase II clinical trials, with ongoing research into its effectiveness and safety.
- Route of Administration:It is being investigated for oral, topical, and vaginal administration.
- Research and Development:Pritelivir is being developed by AiCuris Anti-infective Cures, building upon research from Bayer.
Pritelivir (development codes AIC316 or BAY 57-1293) is a direct-acting antiviral drug in development for the treatment of herpes simplex virus infections (HSV). This is particularly important in immune compromised patients. It is currently in Phase III clinical development by the German biopharmaceutical company AiCuris Anti-infective Cures AG. US FDA granted fast track designation for pritelivir in 2017 and breakthrough therapy designation 2020.
SCHEME
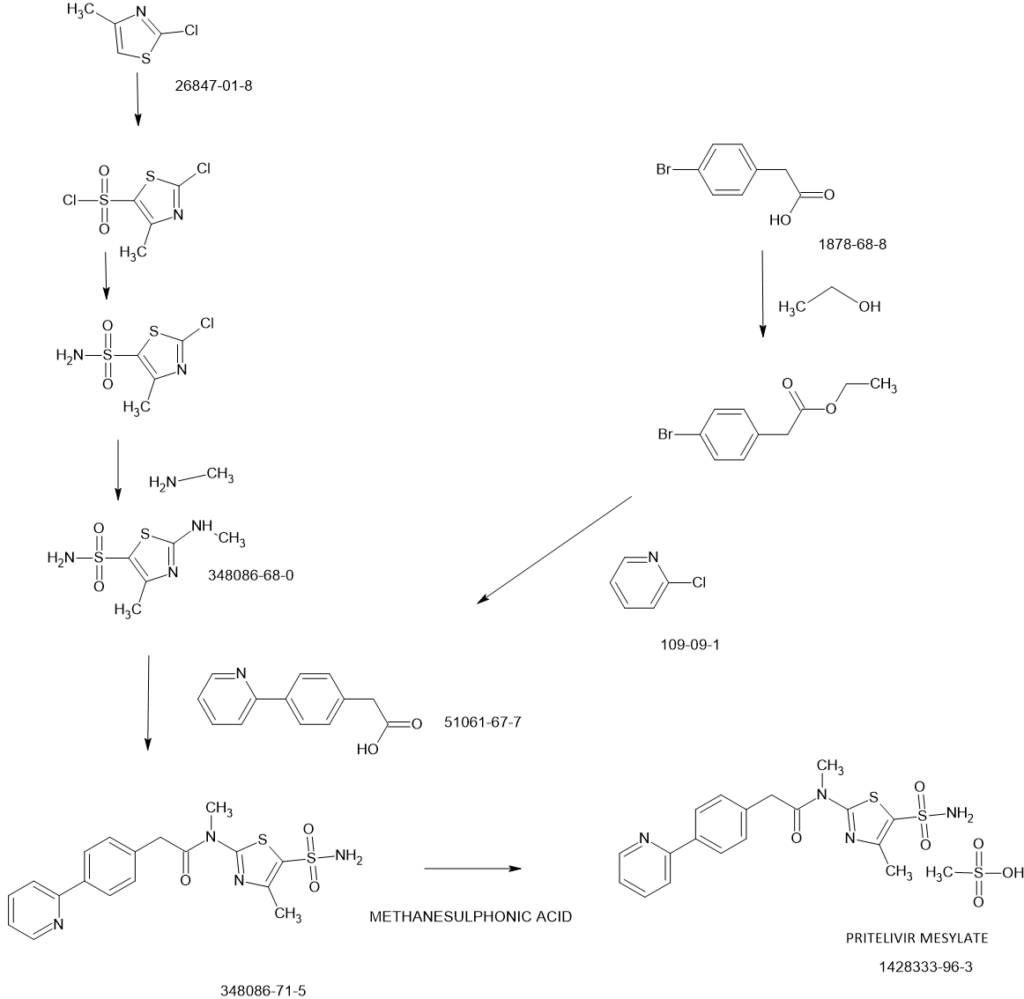
Pritelivir mesylate, an antiviral drug used to treat herpes simplex virus (HSV) infections, is synthesized through a series of chemical reactions, including palladium-catalyzed coupling, ester saponification, and amide coupling reactions. The mesylate salt is then formed by reacting the free base with methanesulfonic acid.
Detailed Synthesis Steps:
- 1. Diaryl Acetic Acid Synthesis:Diaryl acetic acid reagents are synthesized using palladium-catalyzed coupling reactions. These reactions involve the use of organometallic intermediates derived from halo-aryl esters.
- 2. Ester Saponification:The ester group in the synthesized compounds is then converted to a carboxylic acid group through saponification.
- 3. Amide Coupling:The resulting carboxylic acids are coupled with thiazolyl sulfonamides using amide coupling conditions to form the pritelivir molecule.
- 4. Salt Formation:The pritelivir free base is then reacted with methanesulfonic acid to form the mesylate salt, which is the active pharmaceutical ingredient (API).
Key Aspects of Pritelivir Mesylate Synthesis:
- Targeted Mechanism:Pritelivir mesylate inhibits the herpes simplex virus by targeting the viral helicase-primase complex, essential for DNA replication, unlike traditional antivirals that target DNA polymerase.
- Salt and Polymorph Screening:An extensive salt and polymorph screening is performed to optimize the pharmaceutical development of pritelivir, resulting in various salt forms including the mesylate, maleate, and sulfate.
- Solubility and Stability:Pritelivir mesylate is a BCS Class II drug substance with pH-dependent solubility. It exhibits high solubility below pH 3 and poor solubility at neutral pH.
- Formulation Considerations:Due to its limited water solubility, pritelivir mesylate is often formulated with solvents like DMSO, PEG300, Tween-80, and saline or with cyclodextrins like SBE-β-CD.
- Clinical Trials:Pritelivir mesylate is currently under extensive study to evaluate its efficacy and safety profile, with promising results in early clinical trials.
PATENT
WO2018096170
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018096170&_cid=P20-MCVCP5-34284-1
PATENT
WO2018096177
PATENT
https://patents.google.com/patent/WO2018096177A1/en
Likewise, EP 2 598 502 Al describes the crystalline mono mesylate monohydrate salt of N- [5-(aminosulfonyl)-4-methyl- 1 ,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]-acetar^ in a definite particle size distribution and a specific surface area range, which has demonstrated increased long term stability and release kinetics from pharmaceutical compositions, as well as to pharmaceutical compositions containing said N-[5- (aminosulfonyl)-4-methyl- 1 ,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetaniide mono mesylate monohydrate having the afore-mentioned particle size distribution and specific surface area range.
WO 2013/045479 Al describes an improved and shortened synthesis process of N-[5- (ammosulfonyl)-4-methyl-l,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetaniide and the mesylate salt thereof by using boronic acid derivatives or borolane reagents while avoiding toxic organic tin compounds. Moreover, also the crystalline mesylate monohydrate salt of N- [5 -(aminosulfonyl)-4-methyl- 1 ,3 -thiazol-2-yl] -N-methyl-2- [4-(2-pyridinyl)-phenyl] – acetamide is described therein with increased long-term stability and release kinetics from pharmaceutical compositions thereof.
Said pritelivir is an innovative, highly active and specific inhibitor of herpes simplex virus (HSV) infections. As a compound derived from the chemical class of thiazolylamides, pritelivir is active against both types of herpes simplex virus causing labial and genital herpes, respectively, and retains activity against viruses which have become resistant to marketed drugs. Pritelivir has a mode of action that is distinct from other antiviral agents currently in use for treatment of HSV infections (i.e., the nucleoside analogues acyclovir and its prodrug valacyclovir as well as famciclovir, the prodrug of penciclovix). Whereas nucleoside analogs terminate ongoing DNA chain elongation through inhibition of viral DNA polymerase, pritelivir prevents de novo synthesis of virus DNA through inhibition of the helicase-primase complex. In addition, it does not require activation within an HSV infected cell by viral thymidine kinase and therefore, is also protective to uninfected cells.
With the context of the invention, similar expressions which all would denote the compound pritelivir are “BAY 57-1293”, “AIC090096” and “AIC316”.
Likewise, the terms ”pritelivir”, “BAY 57-1293”, “AIC090096” and “AIC316″ or the compound *’N-[5-(ammosulfonyl)-4-methyl-l,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridm phenyl] -acetamide” would reflect throughout the text a compound having the structural formula:

Synthetic route – Manufacture of N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-Yl]-N-methyl- 2-[4-(2-pyridinyl)-phenyl]-acetamide free base hemihydrate
The starting materials (4-pyridine-2-yl-phenyl)-acetic acid (PP-acetic acid; C-023930) and aminothiazole sulfonic acid amide (C-023936) are coupled using standard reaction conditions
(N-Ethyl-N’-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC x HCl), tetrahydrofuran (THF)/N-methylpyrrolidone (NMP) to deliver N-[5-(aminosulfonyl)-4- methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)-phenyl]-acetamide free base hemihydrate (C-023931). To obtain the hemihydrate, N-[5-(aminosulfonyl)-4-methyl-1,3- thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)-phenyl]-acetamide hemihydrate free base is recrystallized from THF/water. A flowchart showing the synthesis of N-[5-(aminosulfonyl)- 4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)-phenyl]-acetamide is provided in below in the reaction scheme below 1.
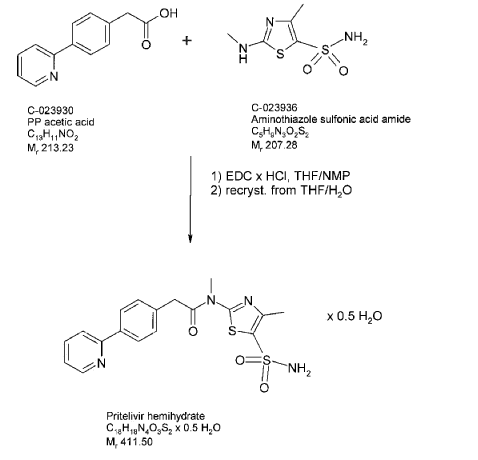
Description of the manufacturing process of N-r5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)-phenyl]-acetamide free base hemihydrate
PP-acetic acid and aminothiazole sulfonic acid amide are mixed in THF/NMP, the mixture is cooled and then EDC x HCl is added in portions. The reaction mixture is stirred for several hours, and then added slowly to purified water. The suspension is stirred and filtered; the product cake is washed with purified water and dried at room temperature in a nitrogen stream and then under vacuum. Purified water is added slowly at elevated temperature, the suspension is stirred for several hours. The suspension is cooled to 5°C and stirred further for several hours. The product is isolated by filtration and washed with purified water. The product is dried at 65°C under vacuum until the criterion for water content is reached. A major advantage of the synthesis of free base of N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)-phenyl]-acetamide hemihydrate is the absence impurities related to the presence of mesylate ester that might be present in the N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)-phenyl]-acetamide mesylate.
PAPER
By: Carta, Fabrizio ; et al. Journal of Medicinal Chemistry (2017), 60(7), 3154-3164
SULPHURIC ACID FOR SULPHATE A SIMILAR REACTION BUT NOT SAME
PAPER
https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c00668
Chemistry of Pritelivir
Synthesis of pritelivir and its analogues is based on the reported methods in the literature (18−20) and presented in Figure 4. A simple retrosynthetic disconnection of the target compound suggests a coupling of the thiazolyl sulfonamide and diaryl acetic acid (Figure 4a). During the course of development an optimized route applying the principles of green chemistry was developed and will be used for the commercial phase.
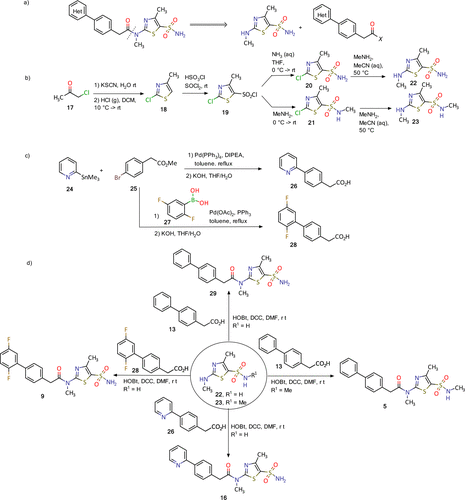
Figure 4. Synthesis of pritelivir (16) and analogues: (a) disconnection approach to target molecules; (b) synthesis of thiazolyl sulfonamide reagents; (c) synthesis of diaryl acetic acids; (d) synthesis of some representative examples of pritelivir and analogues. (18−20)
The synthesis of the thiazolyl sulfonamide reagents begins with a reaction of chloroacetone (17) and potassium thiocyanide to give an intermediate ketone which was cyclized to the thiazole 18 by treatment with gaseous hydrochloride (Figure 4b). Chlorosulfonylation with chlorosulfonic acid and thionyl chloride resulted in the sulfonyl chloride 19 that was converted to the corresponding sulfonamides 20 and 21 after treatment with ammonia or methylamine, respectively. The 2-chloro substituent in 20 and 21 was converted to the methyl amine in an SNAr reaction to deliver the building blocks 22–23. Diaryl acetic acid reagents were synthesized using palladium catalyzed coupling reactions with organometallic intermediates formed from the corresponding halo-aryl esters (Figure 4c). Ester saponification then delivered the corresponding carboxylic acids (e.g., 26 and 28, Figure 4c). Finally, the target molecules, for instance, 5, 11, 16, and 9, were obtained using amide coupling reaction conditions with corresponding diaryl acetic acids and the thiazolyl sulfonamides (Figure 4d). (18−20)
Medical use
Pritelivir is currently being developed for the treatment of immunocompromised patients with mucocutaneous HSV lesions that are resistant to acyclovir.
HSV in immunocompromised patients
Although HSV infection is very common in the general population, it rarely causes serious disease and is effectively contained by the immune system. In those with a weakened immune system such as transplant recipients, people receiving chemo- or radiotherapy, or HIV patients, an active HSV infection can cause disease in 35-68% of patients and may become severe or even life-threatening.[1]
Standard of care treatments
HSV treatment revolves around the use of nucleoside analogues (NA) which act via the viral DNA polymerase, causing DNA chain termination and prevention of viral replication. First-line treatment is generally acyclovir or its prodrug valacyclovir. Resistance to acyclovir is more common in HSV patients with weakened or suppressed immune systems, affecting between 4 and 25% of cases.[2][3][4][5][6]
Resistance to standard treatments
If HSV drug resistance is mediated by mutation(s) of the viral UL23 gene, which encodes the viral thymidine kinase (TK), then the pyrophosphate analogue foscarnet may be effective as a rescue treatment, since it does not require activation by TK. The use of foscarnet is commonly accompanied by restrictive toxicity, particularly nephrotoxicity.[7] If the virus also acquires resistance to foscarnet, then there is currently no FDA approved treatment.
Clinical research
Completed phase II clinical trials in otherwise healthy patients with genital herpes
- A Double-blind Randomized Placebo Controlled Dose-finding Trial to Investigate Different Doses of a New Antiviral Drug in Subjects With Genital HSV Type 2 Infection.[8][9]
- A Double-blind, Double Dummy, Randomized Crossover Trial to Compare the Effect of “AIC316 (Pritelivir)” 100 mg Once Daily Versus Valacyclovir 500 mg Once Daily on Genital HSV Shedding in HSV-2 Seropositive Adults.[10][11]
Ongoing phase II / phase III clinical trials with pritelivir
A phase II / III multinational, comparator-controlled, clinical trial in immunocompromised patients with acyclovir-resistant mucocutaneous lesions is listed on ClinicalTrials.gov[12] and EudraCT.[13]
Pharmacology
Mechanism of action
Pritelivir is a member of the helicase-primase inhibitors (HPI), a novel class of direct-acting antiviral drugs acting specifically against HSV-1 and HSV-2.[14][15] As the name suggests, the drugs act through inhibition of the viral helicase primase complex, encoded by the UL5 (helicase), UL8 (scaffold protein) and UL52 (primase) genes, which is essential for HSV replication.[16] The helicase primase complex is encoded separately from the viral DNA polymerase (encoded by the UL30 gene). Because HPIs i) do not target the viral DNA polymerase and ii) do not require activation by the viral thymidine kinase enzyme (encoded by the UL23 gene), mutations causing resistance to NAs are not protective against HPIs. Similarly, resistance to HPIs does not confer resistance to NAs.
References
- ^ Wilck, M.B.; Zuckerman, R.A.; A. S. T. Infectious Diseases Community of Practice (2013). “Herpes simplex virus in solid organ transplantation”. Am J Transplant. 13 (Suppl 4): 121–7. doi:10.1111/ajt.12105. PMID 23465005. S2CID 44969727.
- ^ Zuckerman, R.; Wald, A.; A. S. T. Infectious Diseases Community of Practice (2009). “Herpes simplex virus infections in solid organ transplant recipients”. Am J Transplant. 9 (Suppl 4): S104-7. doi:10.1111/j.1600-6143.2009.02900.x. PMID 20070669. S2CID 205846431.
- ^ Frobert, E.; Burrel, S.; Ducastelle-Lepretre, S.; Billaud, G.; Ader, F.; Casalegno, J.S. (2014). “Resistance of herpes simplex viruses to acyclovir: an update from a ten-year survey in France”. Antiviral Res. 111: 36–41. doi:10.1016/j.antiviral.2014.08.013. PMID 25218782.
- ^ Patel, D.; Marchaim, D.; Marcus, G.; Gayathri, R.; Lephart, P.R.; Lazarovitch, T.; Zaidenstein, R.; Chandrasekar, P. (2014). “Predictors and outcomes of acyclovir-resistant herpes simplex virus infection among hematopoietic cell transplant recipients: case-case-control investigation”. Clin Transplant. 28 (1): 1–5. doi:10.1111/ctr.12227. PMID 24033498. S2CID 37729458.
- ^ Danve-Szatanek, C.; Aymard, M.; Thouvenot, D.; Morfin, F.; Agius, G.; Bertin, I. (2004). “Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up”. J Clin Microbiol. 42 (1): 242–9. doi:10.1128/JCM.42.1.242-249.2004. PMC 321677. PMID 14715760.
- ^ Chakrabarti, R.; Pillay, D.; Ratcliffe, D.; Cane, P.A.; Collingham, K.E.; Milligan, D.W. (2000). “Resistance to antiviral drugs in herpes simplex virus infections among allogeneic stem cell transplant recipients: risk factors and prognostic significance”. J Infect Dis. 181 (6): 2055–8. doi:10.1086/315524. PMID 10837192.
- ^ SmPC
- ^ NCT01047540
- ^ Wald, A.; Timmler, B.; Magaret, A.; Warren, T.; Trying, S. (2014). “Helicase-primase inhibitor pritelivir for HSV-2 infection”. N Engl J Med. 370 (3): 201–10. doi:10.1056/NEJMoa1301150. PMID 24428466.
- ^ NCT01658826
- ^ Wald, A.; Timmler, B.; Warren, T.; Trying, S.; Johnston, C. (2016). “Effect of Pritelivir Compared With Valacyclovir on Genital HSV-2 Shedding in Patients With Frequent Recurrences: A Randomized Clinical Trial”. JAMA. 316 (23): 2495–2503. doi:10.1001/jama.2016.18189. hdl:1805/14200. PMID 27997653.
- ^ NCT03073967
- ^ 2020-004940-27
- ^ Biswas, S.; Jennens, L.; Field, H.J. (2007). “Single amino acid substitutions in the HSV-1 helicase protein that confer resistance to the helicase-primase inhibitor BAY 57-1293 are associated with increased or decreased virus growth characteristics in tissue culture”. Arch Virol. 152 (8): 1489–500. doi:10.1007/s00705-007-0964-7. PMID 17404685. S2CID 23688945.
- ^ Field, H.J.; Biswas, S. (2011). “Antiviral drug resistance and helicase-primase inhibitors of herpes simplex virus”. Drug Resist Updat. 14 (1): 45–51. doi:10.1016/j.drup.2010.11.002. PMID 21183396.
- ^ Crute, J.J.; Tsurumi, T.; Zhu, L.A.; Weller, S.K.; Olivo, P.D.; Challberg, M.D. (1989). “Herpes simplex virus 1 helicase-primase: a complex of three herpes-encoded gene products”. Proc. Natl. Acad. Sci. U.S.A. 86 (7): 2186–2189. Bibcode:1989PNAS…86.2186C. doi:10.1073/pnas.86.7.2186. PMC 286876. PMID 2538835.
- [1]. Ligat G, et al. Identification of Amino Acids Essential for Viral Replication in the HCMV Helicase-PrimaseComplex. Front Microbiol. 2018 Oct 23;9:2483. [Content Brief][2]. Wald A, et al. Helicase-primase inhibitor Pritelivir for HSV-2 infection. N Engl J Med. 2014 Jan 16;370(3):201-10. [Content Brief][3]. Quenelle DC, et al. Efficacy of pritelivir and acyclovir in the treatment of herpes simplex virus infections in a mouse model of herpes simplex encephalitis. Antiviral Res. 2018 Jan;149:1-6. [Content Brief]
| Names | |
|---|---|
| Systematic IUPAC nameN-Methyl-N-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-[4-(pyridin-2-yl)phenyl]acetamide | |
| Identifiers | |
| CAS Number | 348086-71-5 |
| 3D model (JSmol) | Interactive image |
| ChemSpider | 430613 |
| KEGG | D12811 |
| PubChem CID | 491941 |
| UNII | 07HQ1TJ4JE |
| CompTox Dashboard (EPA) | DTXSID70188344 |
| showInChI | |
| showSMILES | |
| Properties | |
| Chemical formula | C18H18N4O3S2 |
| Molar mass | 402.49 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).Infobox references | |
///////////PRITELIVIR MESYLATE, AIC316 mesylate hydrate, BAY 57-1293 mesylate hydrate, AIC 316 mesylate hydrate, BAY 57-1293 mesylate hydrate, BAY57-1293, BAY 57-1293, BAY-57-1293, BAY571293, BAY 571293, BAY-571293, AIC-316, AIC 316














