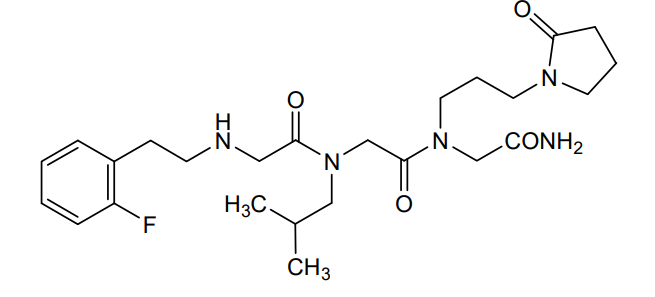
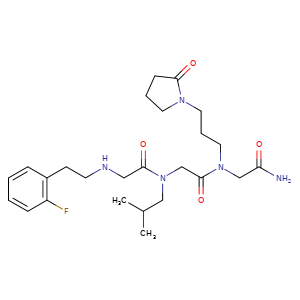
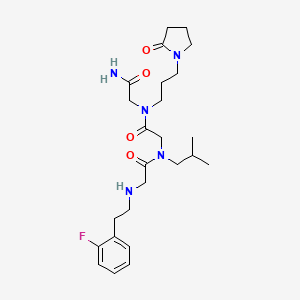
Privosegtor
CAS 1361200-34-1
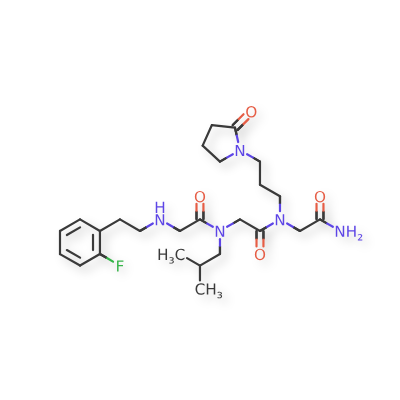
MF C25H38FN5O4, MW 491.6 g/mol
GLYCINAMIDE, N-(2-(2-FLUOROPHENYL)ETHYL)GLYCYL-N-(2-METHYLPROPYL)GLYCYL-N2-(3-(2-OXO-1-PYRROLIDINYL)PROPYL)-
N-(2-(2-FLUOROPHENYL)ETHYL)GLYCYL-N-(2-METHYLPROPYL)GLYCYL-N2-(3-(2-OXO-1-PYRROLIDINYL)PROPYL)GLYCINAMIDE
N-(2-(2-FLUOROPHENYL)ETHYL)GLYCYL-N-(2-METHYLPROPYL)GLYCYL-N2-(3-(2-OXOPYRROLIDIN-1-YL)PROPYL)GLYCINAMIDE
N-[2-(2-fluorophenyl)ethyl]glycyl-N-(2-methylpropyl)glycyl-N2[3-(2-oxopyrrolidin-1-yl)propyl]glycinamide
serum/ glucocorticoid-regulated kinase 2 (Sgk2) activator, Phase 2, Optic neuritis, orphan drug, BN-201, BN 201, G-79, G 79, KCN37L7EIH
- OriginatorBionure
- DeveloperBionure; Oculis Pharma
- ClassAnti-inflammatories; Antiglaucomas; Eye disorder therapies; Neuroprotectants; Peptides; Small molecules
- Mechanism of ActionBrain derived neurotrophic factor agonists; Insulin-like growth factor I stimulants; Neuron modulators; Serum-glucocorticoid regulated kinase stimulants
- Orphan Drug StatusYes – Optic neuritis
- Phase IIOptic neuritis
- PreclinicalMultiple sclerosis; Neurotrophic keratopathy
- No development reportedGlaucoma; Neuromyelitis optica
- 06 Oct 2025Oculis Holding plans the PIONEER-2 trial in Optic neuritis in first half of 2026
- 06 Oct 2025Oculis Holding plans the PIONEER-3 trial in Optic nerve disorders in mid-2026
- 06 Oct 2025Oculis Holding completes End-of-phase II meeting with US FDA and receives positive feedback for registrational PIONEER program in Optic neuritis and Optic nerve disorders
OCS-05 in Patients With Optic Neuritis
CTID: NCT04762017
Phase: Phase 2
Status: Completed
Date: 2025-09-22
N-[2-[(2-amino-2-oxoethyl)-[3-(2-oxopyrrolidin-1-yl)propyl]amino]-2-oxoethyl]-2-[2-(2-fluorophenyl)ethylamino]-N-(2-methylpropyl)acetamide (BN201) is a small peptide molecule, a first-in-class neuroprotective compound. BN201 promotes the survival of cultured neural cells when subjected to oxidative stress or when deprived of trophic factors. BN201 promotes neuronal differentiation, the differentiation of precursor cells to mature oligodendrocytes in vitro, and the myelination of new axons. BN201 modulates several kinases participating in the insulin growth factor 1 pathway including serum-glucocorticoid kinase and midkine, inducing the phosphorylation of NDRG1 and the translocation of the transcription factor Foxo3 to the cytoplasm. In vivo, BN201 prevents axonal and neuronal loss, and it promotes remyelination in models of multiple sclerosis, chemically induced demyelination, and glaucoma. Bionure, a spin-off from Hospital Clínic de Barcelona that is based in California, is developing BN201 for multiple sclerosis, acute optic neuritis (AON) and glaucoma. BN201 was granted with orphan designation status for optic neuritis by the FDA. Optic neuritis is often an early sign of multiple sclerosis. The efficacy, safety, and capacity of the drug to cross the blood-brain barrier have been demonstrated in animal models, but the drug has not yet entered clinical testing.
PAT
Agonists of neurotrophin receptors and their use as medicaments
Publication Number: WO-2012028959-A1
Priority Date: 2010-08-31
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012028959&_cid=P10-MIDYQ0-58943-1
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021084013&_cid=P10-MIDYSN-60542-1
In another embodiment, optionally in combination with one or more features of the various embodiments described above or below throughout all the description, the compound of formula (I) is selected from the group consisting of G79 ([N-(2-(2′-fluorophenyl)ethyl)- glycyl]-[N-(2-methylpropyl)-glycyl]-N-[3-(2′-oxopyrrolidinyl)-propyl]glycinamide, BN201 , Chemical Formula: C25H38FN5O4; MW 491.5987), G-80 ([N-(2-(2′-fluorophenyl)ethyl)- glycyl]-[N-(2-methyl-propyl)glycyl]-N-[2-(4′-sulfamoyl-phenyl)ethyl]glycinamide, BN 119, Chemical Formula: C26H36FN5O5S; MW 549.658) and G81 ([N-(2-(1 -pyrrolidinyl)ethyl)- glycyl]-[N-(2-methyl-propyl)glycyl]-N-[2-(4′-sulfamoyl-phenyl)ethyl]glycinamide, BN 120, Chemical Formula: C24H4oN6OS; MW 524.6766):
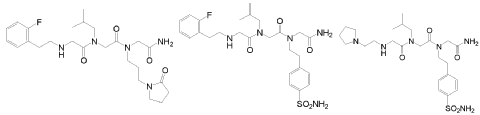
G79 (BN201) G80 (BN119) G81 (BN120)
Compounds of formula (I) can be prepared as disclosed in WO2012028959.
PAT
- Agonists of Neurotrophin Receptors and Their Use as MedicamentsPublication Number: US-2012052094-A1Priority Date: 2010-08-31
- Agonists of Neurotrophin Receptors and Their Use as MedicamentsPublication Number: US-2015005239-A1Priority Date: 2010-08-31
- Agonists of neurotrophin receptors and their use as medicamentsPublication Number: US-2017121367-A1Priority Date: 2010-08-31
- Agonists of neurotrophin receptors and their use as medicamentsPublication Number: US-8791076-B2Priority Date: 2010-08-31Grant Date: 2014-07-29
- Agonists of neurotrophin receptors and their use as medicamentsPublication Number: US-9453047-B2Priority Date: 2010-08-31Grant Date: 2016-09-27
- Combination Therapy Methods, Compositions and KitsPublication Number: KR-20220109378-APriority Date: 2019-07-03
- Combination therapy methods, compositions and kitsPublication Number: US-2022378866-A1Priority Date: 2019-07-03
- Agonists of neurotrophin receptors and their use as medicamentsPublication Number: EP-2611775-A1Priority Date: 2010-08-31
- Agonists of neurotrophin receptors and their use as medicamentsPublication Number: EP-2611775-B1Priority Date: 2010-08-31Grant Date: 2016-03-16
- Agonists of neurotrophin receptors and their use as medicamentsPublication Number: US-10106577-B2Priority Date: 2010-08-31Grant Date: 2018-10-23
- Combination therapy methods, compositions and kitsPublication Number: WO-2021001464-A1Priority Date: 2019-07-03
- Combination therapy methods, compositions and kitsPublication Number: AU-2020298782-A1Priority Date: 2019-07-03
- Combination therapy methods, compositions and kitsPublication Number: CN-114206329-APriority Date: 2019-07-03
- Combination therapy methods, compositions and kitsPublication Number: EP-3993784-A1Priority Date: 2019-07-03
- Combination therapy methods, compositions and kitsPublication Number: JP-2022539999-APriority Date: 2019-07-03
- Boron-nitrogen compound, organic electroluminescence composition, and organic electroluminescence device containing samePublication Number: WO-2022121951-A1Priority Date: 2020-12-10
- New treatment regimen for the treatment of neurological diseases or conditionsPublication Number: WO-2021084013-A1Priority Date: 2019-10-30
- Novel Therapeutic Approaches for the Treatment of Neurological Diseases or ConditionsPublication Number: CN-115052595-APriority Date: 2019-10-30
- New treatment regimen for the treatment of neurological diseases or conditionsPublication Number: EP-4051263-A1Priority Date: 2019-10-30
- New treatment regiment for the treatment of neurological diseases or conditionsPublication Number: US-2022387385-A1Priority Date: 2019-10-30
- A plant zinc-increasing compound inoculant and its preparation method and applicationPublication Number: CN-117286034-APriority Date: 2023-09-11
- A plant zinc-enhancing composite bacterial agent and its preparation method and applicationPublication Number: CN-117286034-BPriority Date: 2023-09-11Grant Date: 2024-11-15
- Compound, pharmaceutical composition comprising the same, and process for synthesizing the samePublication Number: TW-202432095-APriority Date: 2022-12-22
- Synthesis of small molecule agonists of neuroptrophinPublication Number: WO-2024133860-A1Priority Date: 2022-12-22
- Boron-nitrogen compound, organic electroluminescent composition and organic electroluminescent device containing samePublication Number: WO-2022121920-A1Priority Date: 2020-12-10



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
- Development and validation of PAMPA-BBB QSAR model to predict brain penetration potential of novel drug candidatesPublication Name: Frontiers in PharmacologyPublication Date: 2023-12-01PMCID: PMC10722238PMID: 38108064DOI: 10.3389/fphar.2023.1291246
- A Phase 1 randomized study on the safety and pharmacokinetics of OCS-05, a neuroprotective disease modifying treatment for Acute Optic Neuritis and Multiple SclerosisPublication Name: Scientific ReportsPublication Date: 2023-03-29PMCID: PMC10060579PMID: 36991169DOI: 10.1038/s41598-023-32278-0
- Retrospective assessment of rat liver microsomal stability at NCATS: data and QSAR modelsPublication Name: Scientific ReportsPublication Date: 2020-11-26PMCID: PMC7693334PMID: 33244000DOI: 10.1038/s41598-020-77327-0
- A High-Throughput Screen of a Library of Therapeutics Identifies Cytotoxic Substrates of P-glycoproteinPublication Name: Molecular PharmacologyPublication Date: 2019-11PMCID: PMC6790066PMID: 31515284DOI: 10.1124/mol.119.115964
- Predictive models of aqueous solubility of organic compounds built on A large dataset of high integrityPublication Name: Bioorganic & Medicinal ChemistryPublication Date: 2019-07-15PMCID: PMC8274818PMID: 31176566DOI: 10.1016/j.bmc.2019.05.037
/////////Privosegtor, Phase 2, Optic neuritis, orphan drug, BN-201, BN 201, G-79, G 79, KCN37L7EIH














