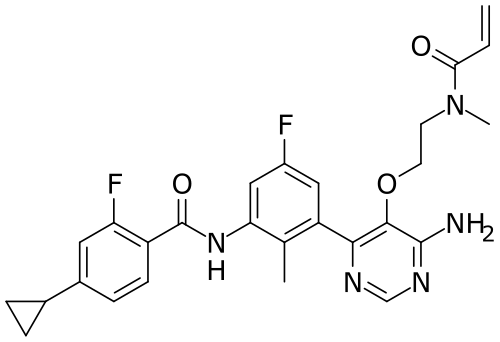
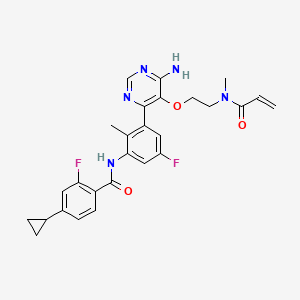
Remibrutinib
CAS 1787294-07-8
N-[3-[6-amino-5-[2-[methyl(prop-2-enoyl)amino]ethoxy]pyrimidin-4-yl]-5-fluoro-2-methylphenyl]-4-cyclopropyl-2-fluorobenzamide
MW 507.5 g/mol, MF C27H27F2N5O3
- LOU064
- NVP-LOU064-NXA
- LOU064-NXA
- I7MVZ8HDNU
- WHO 11062
APPROVALS 2025, FDA 2025, 9/30/2025, To treat chronic spontaneous urticaria in adults who remain symptomatic despite H1 antihistamine treatment
Remibrutinib, sold under the brand name Rhapsido, is a medication used for the treatment of chronic spontaneous urticaria.[1] Remibrutinib is an oral, small molecule kinase inhibitor that inhibits Bruton’s tyrosine kinase (BTK).[1] It is taken by mouth.[1]
SYN
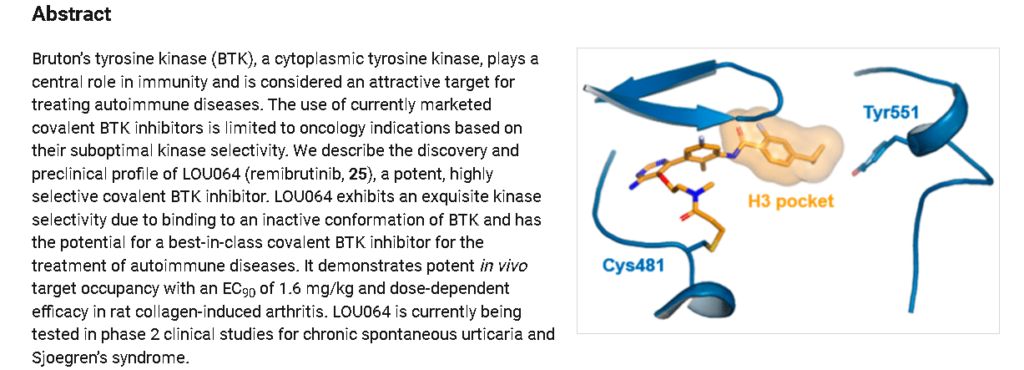
Discovery of LOU064 (Remibrutinib), a Potent and Highly Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase
https://pubs.acs.org/doi/10.1021/acs.jmedchem.9b01916
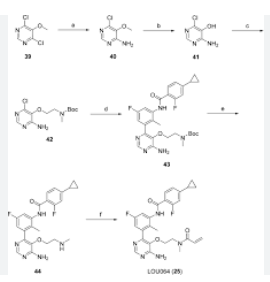
SYN
- WO2015079417
- https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015079417&_cid=P12-MH1R14-25744-1
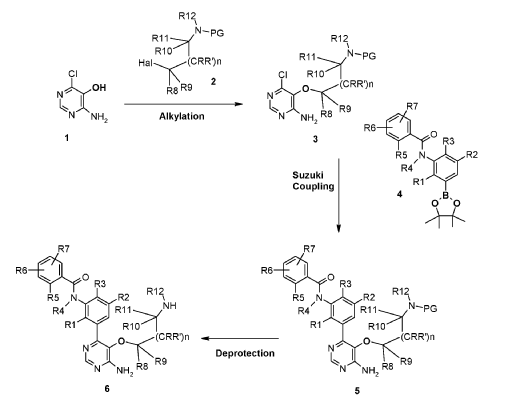
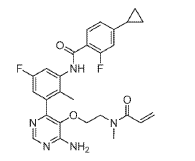
Example 6
N-(3-(6-Amino-5-(2-(N-methylacrylamido)ethoxy)pyrimidin-4-yl)-5-fluoro-2- methylphenyl)-4-cyclopropyl-2-fluorobenzamide
(1) tert-Butyl (2-((4-amino-6-chloropyrimidin-5-yl)oxy)ethyl)(methyl)carbamate, INT 8
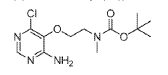
To a solution of 4-amino-6-chloropyrimidin-5-ol (content 90%, 2.00 g, 12.37 mmol) in THF (120 mL) was added N-Boc-N-methyl-2-hydroxyethylamine (6.07 g, 34.64 mmol) followed by SMOPEX-301 (1 mmol/g, 30.90 g, 30.90 mmol). Then, a solution of DIAD (6.01 mL, 30.52 mmol) in THF (20 mL) was added slowly. The reaction mixture was stirred at 60 °C for 3 hr. The mixture was filtered through a pad of Celite. The filtrate was concentrated to afford an oil which was triturated with EtOAc and a white precipitate was formed. The solid was filtered off to afford INT 8. The mother liquor was concentrated and the residue was purified by flash chromatography (silica; DCM/EtOAc gradient, 0- 100%) to afford more INT 8 as a beige solid.
UPLC-MS: MS (ESI): [M+H]+ 303.1, rt = 0.86 min. 1H NMR (DMSO-d6): δ (ppm) 7.97 (s, 1H), 7.26 (s, br, 2H), 4.02-3.93 (m, 2H), 3.54 (t, 2H), 2.89 (s, br, 3H), 1.39 (s, 9H).
(2) tert-Butyl (2-((4-amino-6-(3-(4-cyclopropyl-2-fluorobenzamido)-5-fluoro-2- methylphenyl)pyrimidin-5-yl)oxy)ethyl)(methyl)carbamate, INT 9
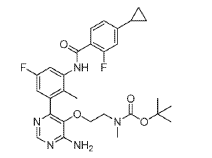
To a solution of INT 8 (447 mg, 1.48 mmol) in DME (7.0 mL) and water (1.0 mL) was added INT 5 (638 mg, 1.54 mmol) followed by aqueous sodium carbonate solution (1 M, 4.21 mL, 4.21 mmol). The mixture was degassed with argon for 10 min and bis(triphenylphosphine)palladium(II) dichloride (49.2 mg, 0.070 mmol) was added. The reaction mixture was stirred at 110 °C for 10 min in a microwave reactor. More INT 5 (232 mg, 0.56 mmol) was added and the reaction mixture was stirred at 110 °C for an additional 15 min in a microwave reactor. The mixture was partitioned between saturated aqueous sodium hydrogen carbonate solution and EtOAc. The organic layer was washed with water and brine, dried over magnesium sulfate, filtered and concentrated. The residue was purified by flash chromatography (silica; DCM/EtOAc gradient, 0-100%) to afford INT 9 as an off-white solid.
UPLC-MS: MS (ESI): [M+H]+ 554.3, rt = 1.21 min. 1H NMR (DMSO-d6): δ (ppm) rotamers 9.76 (s, 1H), 8.19 (s, 1H), 7.74-7.53 (m, 2H) 7.20-6.85 (m, 5H), 3.57-3.48 (m, 2H), 3.29- 3.15 (m, 2H), 2.58 (s, 3H), 2.08-1.99 (overlapping s, 3H and m, 1H), 1.34 and 1.28 (s, 9H), 1.10-1.02 (m, 2H), 0.84-0.77 (m, 2H).
(3) N-(3-(6-Amino-5-(2-(methylamino)ethoxy)pyrimidin-4-yl)-5-fluoro-2- methylphenyl)-4-cyclopropyl-2-fluorobenzamide, INT 10
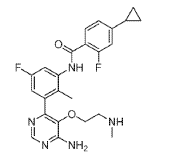
To a solution of INT 9 (335 mg, 0.61 mmol) in DCM (5.0 mL) was added TFA (0.47 mL, 6.05 mmol). The reaction mixture was stirred at RT for 15 hr. The mixture was concentrated under reduced pressure. The residue was dried in vacuum to afford INT 10 as theTFA salt as a brown oil.
UPLC-MS: MS (ESI): [M+H]+ 454.3, rt = 0.73 min. 1H NMR (DMSO-d6): δ (ppm) 10.02 (s, 1H), 9.07-8.13 (s, v br, number of H cannot be assigned), 8.58 (s, 1H), 8.51 (s, br, 2H), 7.71-7.61 (m, 2H), 7.29-7.22 (m, 1H), 7.14-7.05 (m, 2H), 3.75-3.65 (m, 2H), 3.16-3.07 (m, 2H), 2.48 (s, 3H, overlapping with solvent peak), 2.12 (s, 3H), 2.10-1.99 (m, 1H), 1.11-1.03 (m, 2H), 0.83-0.76 (m, 2H).
(4) N-(3-(6-Amino-5-(2-(N-methylacrylamido)ethoxy)pyrimidin-4-yl)-5-fluoro-2-methylphenyl)-4-cyclopropyl-2-fluorobenzamide
To a solution of acrylic acid (62 mg, 0.87 mmol) in DMF (4.0 mL) was added DIPEA (0.302 mL, 1.73 mmol) followed by T3P solution (50% in DMF) (0.438 mL, 0.750 mmol). The mixture was stirred at RT for 30 min. To a solution of INT 10 (containing 3.0 eq TFA, content 90%, 510 mg, 0.577 mmol) and DIPEA (0.302 mL, 1.731 mmol) in DMF (2.0 mL) at 0 °C was added dropwise the above solution. The reaction mixture was stirred at 0 °C for 30 min. The mixture was diluted with water and extracted with EtOAc. The organic layer was washed with water (2x) and brine (2x), dried over magnesium sulfate, filtered and concentrated. The residue was purified by flash chromatography (silica;
DCM/(MeOH with 2% aqueous ammonium hydroxide) gradient, 0-9%) to afford the title compound Example 6 as a white solid.
UPLC-MS: MS (ESI): [M+H]+ 508.3, rt = 0.95 min. 1H NMR (DMSO-d6): δ (ppm) rotamers 9.77 and 9.56 (s, total 1H), 8.25-8.14 (m, 1H), 7.79-7.50 (m, 2H), 7.17-6.93 (m, 5H), 6.70-6.55 (m, 1H), 6.06 (t, 1H), 5.59 (d, 1H), 3.63-3.40 (m, 4H), 2.80 and 2.49 (s, total 3H, peak at 2.49 overlapping with solvent peak), 2.09-1.93 (m, 4H), 1.11-1.00 (m, 2H), 0.85-0.76 (m, 2H).
PAT
- US9512084,
- https://patentscope.wipo.int/search/en/detail.jsf?docId=US133778840&_cid=P12-MH1R60-30520-1
SYN
Publication Date: 2020
Publication Name: Synfacts
PAT
- Amino Pyrimidine DerivativesPublication Number: US-2023312483-A1Priority Date: 2013-11-29
- A complex of bisphenol and a phosphorus compound and heat-developable image-recording material containing the samePublication Number: DE-60121375-T2Priority Date: 2000-01-11Grant Date: 2007-07-05
- Photothermographic materialPublication Number: DE-60011207-T2Priority Date: 1999-10-26Grant Date: 2005-06-23
- CD19 binding molecules and uses thereofPublication Number: US-12221481-B2Grant Date: 2025-02-11



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
Medical uses
Remibrutinib is indicated for the treatment of chronic spontaneous urticaria in adults who remain symptomatic despite H1 antihistamine treatment[1]
Society and culture
Legal status
Remibrutinib was approved for medical use in the United States in September 2025.[2]
Names
Remibrutinib is the international nonproprietary name.[3]
Remibrutinib is sold under the brand name Rhapsido.[2]
References
- https://www.novartis.com/us-en/sites/novartis_us/files/rhapsido.pdf
- “Novartis receives FDA approval for Rhapsido (remibrutinib), the only oral, targeted BTKi treatment for chronic spontaneous urticaria (CSU)” (Press release). Novartis Pharmaceuticals. 30 September 2025. Retrieved 1 October 2025 – via PR Newswire.
- World Health Organization (2020). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83”. WHO Drug Information. 34 (1). hdl:10665/339768.
Further reading
- Maurer, Marcus; Berger, William; Giménez-Arnau, Ana; Hayama, Koremasa; Jain, Vipul; Reich, Adam; et al. (December 2022). “Remibrutinib, a novel BTK inhibitor, demonstrates promising efficacy and safety in chronic spontaneous urticaria”. The Journal of Allergy and Clinical Immunology. 150 (6): 1498–1506.e2. doi:10.1016/j.jaci.2022.08.027. hdl:10230/55511. ISSN 1097-6825. PMID 36096203.
- Maurer, Marcus; Giménez-Arnau, Ana; Jain, Vipul; Tillinghast, Jeffrey; Tolcachier, Alberto; Nigen, Simon; et al. (February 2022). “Remibrutinib Treatment Improves Quality of Life in Patients with Chronic Spontaneous Urticaria”. Journal of Allergy and Clinical Immunology. 149 (2): AB179. doi:10.1016/j.jaci.2021.12.589. S2CID 246522006.
External links
- Clinical trial number NCT05030311 for “A Phase 3 Study of Efficacy and Safety of Remibrutinib in the Treatment of CSU in Adults Inadequately Controlled by H1 Antihistamines (REMIX-1)” at ClinicalTrials.gov
- Clinical trial number NCT05032157 for “A Phase 3 Study of Efficacy and Safety of Remibrutinib in the Treatment of CSU in Adults Inadequately Controlled by H1-antihistamines (REMIX-2)” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Trade names | Rhapsido |
| License data | US DailyMed: Remibrutinib |
| Routes of administration | By mouth |
| ATC code | L04AA60 (WHO) |
| Legal status | |
| Legal status | US: ℞-only[1] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1787294-07-8 |
| PubChem CID | 118107483 |
| IUPHAR/BPS | 10457 |
| DrugBank | DB16852 |
| ChemSpider | 78317000 |
| UNII | I7MVZ8HDNU |
| KEGG | D12285 |
| ChEMBL | ChEMBL4483575 |
| PDB ligand | N6Z (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C27H27F2N5O3 |
| Molar mass | 507.542 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
- Maurer M, Berger W, Gimenez-Arnau A, Hayama K, Jain V, Reich A, Haemmerle S, Lheritier K, Walsh P, Xia S, Storim J: Remibrutinib, a novel BTK inhibitor, demonstrates promising efficacy and safety in chronic spontaneous urticaria. J Allergy Clin Immunol. 2022 Dec;150(6):1498-1506.e2. doi: 10.1016/j.jaci.2022.08.027. Epub 2022 Sep 9. [Article]
- Nuesslein-Hildesheim B, Ferrero E, Schmid C, Huck C, Smith P, Tisserand S, Rubert J, Bornancin F, Eichlisberger D, Cenni B: Remibrutinib (LOU064) inhibits neuroinflammation driven by B cells and myeloid cells in preclinical models of multiple sclerosis. J Neuroinflammation. 2023 Aug 26;20(1):194. doi: 10.1186/s12974-023-02877-9. [Article]
- Bozek A, Reich A: Evaluating remibrutinib in the treatment of chronic spontaneous urticaria. Immunotherapy. 2025 May;17(7):479-484. doi: 10.1080/1750743X.2025.2510892. Epub 2025 Jun 2. [Article]
- Kaul M, End P, Cabanski M, Schuhler C, Jakab A, Kistowska M, Kinhikar A, Maiolica A, Sinn A, Fuhr R, Cenni B: Remibrutinib (LOU064): A selective potent oral BTK inhibitor with promising clinical safety and pharmacodynamics in a randomized phase I trial. Clin Transl Sci. 2021 Sep;14(5):1756-1768. doi: 10.1111/cts.13005. Epub 2021 Apr 9. [Article]
- Gimeno R, Ribas-Llaurado C, Pesque D, Andrades E, Cenni B, Ambros B, Pujol R, Gimenez-Arnau AM: Remibrutinib inhibits hives effector cells stimulated by serum from chronic urticaria patients independently of FcepsilonR1 expression level and omalizumab clinical response. Clin Transl Allergy. 2023 Mar;13(3):e12227. doi: 10.1002/clt2.12227. [Article]
- Dorner T, Kaul M, Szanto A, Tseng JC, Papas AS, Pylvaenaeinen I, Hanser M, Abdallah N, Grioni A, Santos Da Costa A, Ferrero E, Gergely P, Hillenbrand R, Avrameas A, Cenni B, Siegel RM: Efficacy and safety of remibrutinib, a selective potent oral BTK inhibitor, in Sjogren’s syndrome: results from a randomised, double-blind, placebo-controlled phase 2 trial. Ann Rheum Dis. 2024 Feb 15;83(3):360-371. doi: 10.1136/ard-2023-224691. [Article]
- FDA Approved Drug Products: RHAPSIDO (remibrutinib) tablets, for oral use [Link]
- Novartis: Novartis receives FDA approval for Rhapsido® (remibrutinib), the only oral, targeted BTKi treatment for chronic spontaneous urticaria (CSU) [Link]
//////////Remibrutinib, APPROVALS 2025, FDA 2025, Rhapsido, LOU064, NVP-LOU064-NXA, LOU064-NXA, I7MVZ8HDNU, WHO 11062














