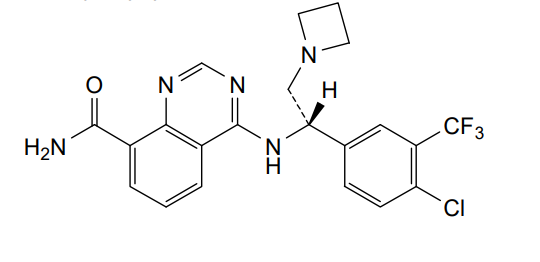
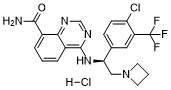
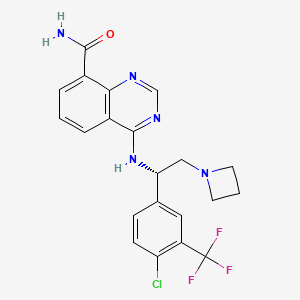
Rupitasertib
CAS 1379545-95-5
MF C21H19ClF3N5O 449.9 g/mol
4-({(1S)-2-(azetidin-1-yl)-1-[4-chloro-3-(trifluoromethyl)phenyl]ethyl}amino)quinazoline-8-carboxamide
4-[[(1S)-2-(azetidin-1-yl)-1-[4-chloro-3-(trifluoromethyl)phenyl]ethyl]amino]quinazoline-8-carboxamide
serine/ threonine kinase inhibitor, antineoplastic, EMD SERONO, Gastric cancer; HER2 positive breast cancer; Solid tumours, M2698 HCl, M2698 hydrochloride, MSC2363318A, MSC 2363318A, MSC-2363318A, M2698, M-269, M 2698. Rupitasertib HCl, 0DXG50I4WD
- OriginatorEMD Serono
- DeveloperEMD Serono; Evexta Bio
- ClassAntineoplastics; Small molecules
- Mechanism of Action70 kDa ribosomal protein S6 kinase inhibitors; Proto-oncogene protein c-akt inhibitors
- PreclinicalGlioblastoma; HER2 negative breast cancer
- No development reportedGastric cancer; HER2 positive breast cancer; Solid tumours
- 28 Oct 2025No recent reports of development identified for preclinical development in Gastric-cancer in France (PO)
- 28 Jun 2025No recent reports of development identified for phase-I development in HER2-positive-breast-cancer(Combination therapy, Late-stage disease, Metastatic disease) in USA (PO)
- 28 Jun 2025No recent reports of development identified for phase-I development in Solid-tumours(Combination therapy, Late-stage disease) in USA (PO)
- First-in-Human Dose Escalation Trial in Subjects With Advanced Malignancies
- CTID: NCT01971515
- Phase: Phase 1
- Status: Completed
- Date: 2018-09-19
Rupitasertib is an orally available inhibitor of the serine/threonine protein kinases ribosomal protein S6 Kinase (p70S6K) and Akt (protein kinase B), with potential antineoplastic activity. Upon administration, rupitasertib binds to and inhibits the activity of p70S6K and Akt. This prevents the activation of the PI3K/Akt/p70S6K signaling pathway and inhibits tumor cell proliferation in cancer cells that have an overactivated PI3K/Akt/p70S6K signaling pathway. Constitutive activation and dysregulated signaling of the PI3K/Akt/p70S6K pathway are frequently associated with tumorigenesis of many tumor types; targeting multiple kinases in this pathway is more efficacious than targeting a single kinase.
An optimized S6K inhibitor to overcome limitations of PAM pathway inhibitors
In just over 20 years, protein kinase inhibitors have changed the face of oncology and opened the new eras of targeted therapies and precision medicine. However, with few exceptions, no patient can be cured by one of these drugs alone. Today, scientists seek to develop novel kinase inhibitors[1] with improved efficacy and the potential to overcome resistances. The dual S6K AKT1/3 inhibitor rupitasertib (formerly DIACC3010, acquired from Merck KGaA, Darmstadt, Germany) has both of these characteristics and reaches brain metastases. After successfully completing a Phase I trial in patients with advanced/refractory solid tumors, including breast cancer, the drug candidate will be evaluated in a Phase 2/3 trial in ER+ HER2 breast cancer, which is expected to start in 2024.
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012069146&_cid=P10-MIJPKI-12294-1
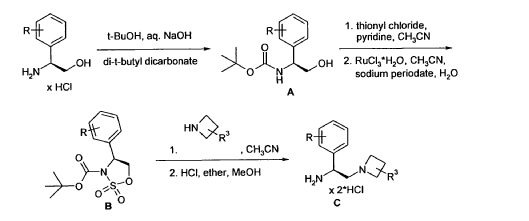
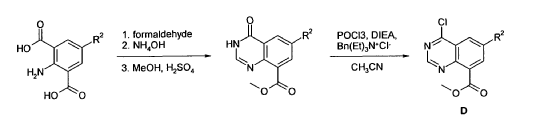
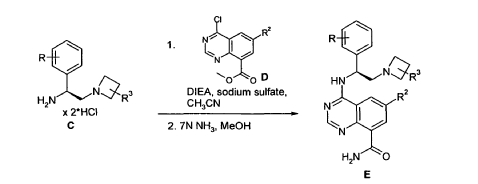
Example 4 was prepared following the general synthesis of A-E starting with (S)-2- amino-2-(3,4-di-fluoro-phenyl)-ethanol.LCMS [384.20 (M+1)]. 1H NMR (DMSO-d6, ppm) 1.92 (2H), 2.75 (1H), 2.93 (1H), 3.15 (4H), 5.43 (1H), 7.34 (2H), 7.53 (1H), 7.68 (1H), 7.81 (1H), 8.58 (4H), 10.30 (1H).
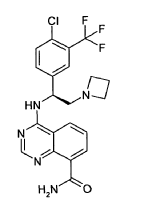
4-[(S)-2-Azetidin-1-yl-1-(4-chloro-3-trifluoromethylphenyl)-ethylamino]-guinazoline-8- carboxylic acid amide (5)
IC50 P70S6K [nM]: 0.9
pS6 MDA-MB-468 [nM]: 11
Akt1 IC50 [nM]: 1.4
Aurora B IC50 [nM]: 100
PAT
- Quinazoline carboxamide azetidinesPublication Number: SG-190318-A1Priority Date: 2010-11-24
- Quinazoline carboxamide azetidinesPublication Number: US-2013252942-A1Priority Date: 2010-11-24
- Quinazoline carboxamide azetidinesPublication Number: US-8946247-B2Priority Date: 2010-11-24Grant Date: 2015-02-03
- SMAC Mimetic for Treating Myelodysplastic SyndromesPublication Number: US-2015158908-A1Priority Date: 2009-07-02
- Methods of treating a ras protein-related disease or disorderPublication Number: US-2025049810-A1



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
- p70S6K/Akt dual inhibitor DIACC3010 is efficacious in preclinical models of gastric cancer alone and in combination with trastuzumabPublication Name: Scientific ReportsPublication Date: 2023-09-25PMCID: PMC10520030PMID: 37749105DOI: 10.1038/s41598-023-40612-9
- TTD: Therapeutic Target Database describing target druggability informationPublication Name: Nucleic Acids ResearchPublication Date: 2023-09-15PMCID: PMC10767903PMID: 37713619DOI: 10.1093/nar/gkad751
- Identification of Clinical Candidate M2698, a Dual p70S6K and Akt Inhibitor, for Treatment of PAM Pathway-Altered CancersPublication Name: Journal of Medicinal ChemistryPublication Date: 2021-10-01PMID: 34596404DOI: 10.1021/acs.jmedchem.1c01087
- Phase 1 study of M2698, a p70S6K/AKT dual inhibitor, in patients with advanced cancerPublication Name: Journal of Hematology & OncologyPublication Date: 2021-08-18PMCID: PMC8371902PMID: 34407844DOI: 10.1186/s13045-021-01132-z
- M2698 is a potent dual-inhibitor of p70S6K and Akt that affects tumor growth in mouse models of cancer and crosses the blood-brain barrierPublication Name: American journal of cancer researchPublication Date: 2016PMCID: PMC4859885PMID: 27186432
////////////Rupitasertib, antineoplastic, EMD SERONO, Gastric cancer; HER2 positive breast cancer; Solid tumours, M2698 HCl, M2698 hydrochloride, MSC2363318A, MSC 2363318A, MSC-2363318A, M2698, M-269, M 2698. Rupitasertib HCl, 0DXG50I4WD














