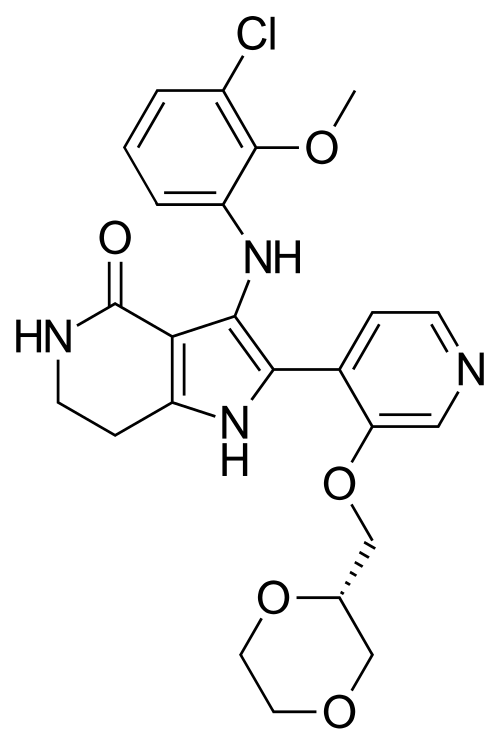
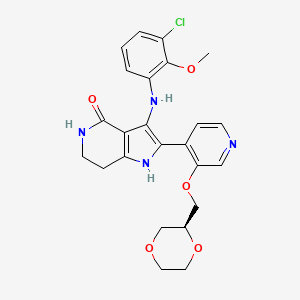
Sevabertinib
CAS 2521285-05-0
MF C24H25ClN4O5, 484.9 g/mol
3-(3-chloro-2-methoxyanilino)-2-[3-[[(2S)-1,4-dioxan-2-yl]methoxy]-4-pyridinyl]-1,5,6,7-tetrahydropyrrolo[3,2-c]pyridin-4-one
11/19/2025, FDA 2025, APPROVALS 2025, Hyrnuo, 2A7VPM5RWH, BAY-2927088, BAY 2927088
To treat locally advanced or metastatic non-squamous non-small cell lung cancer with tumors that have activating HER2 tyrosine kinase domain activating mutations in patients who received a systemic therapy
Sevabertinib, sold under the brand name Hyrnuo, is an anti-cancer medication used for the treatment of non-small cell lung cancer.[1] Sevabertinib is a kinase inhibitor.[1] It is taken by mouth.[1]
Sevabertinib was approved for medical use in the United States in November 2025.[2]
SYN
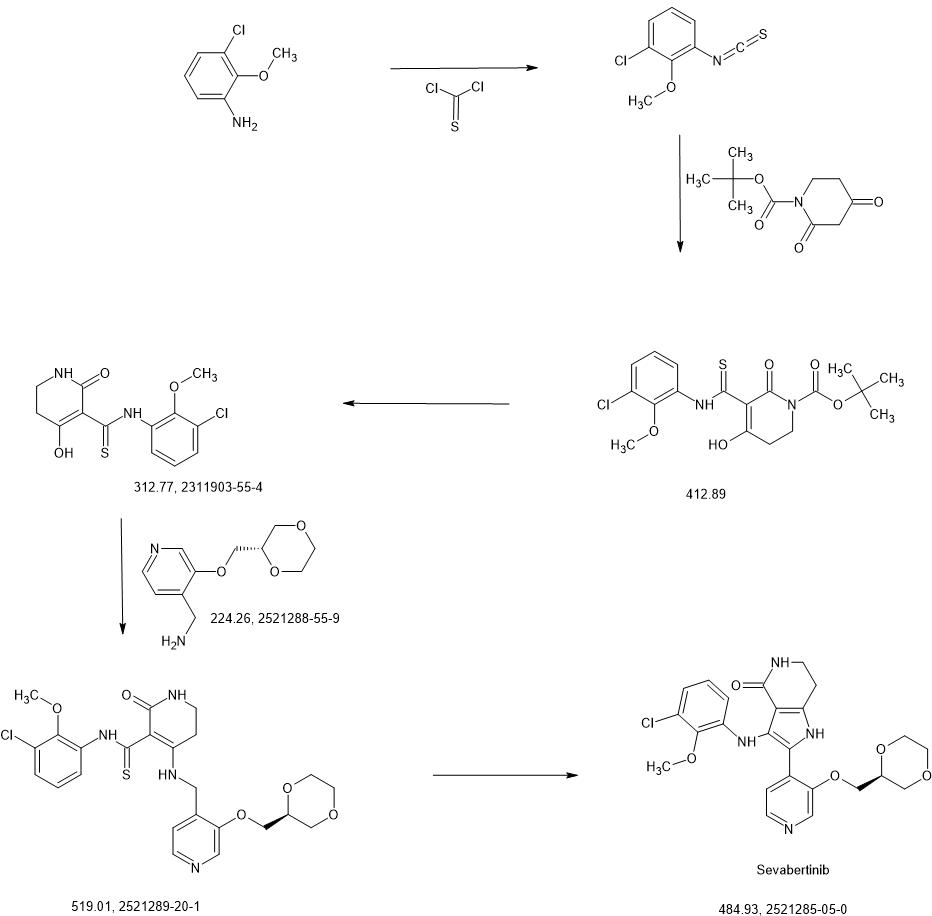
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020216781&_cid=P22-MICIVF-33261-1
Intermediate 3-1
1-chloro-3-isothiocyanato-2-methoxybenzene
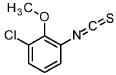
3-chloro-2-methoxyaniline (CAS 511 14-68-2, 8.4 ml, 63 mmol) was solved in DCM (100 ml) and sat. sodium bicarbonate solution (100 ml) was added. To the ice cooled mixture was slowly added thiophosgene (5.4 ml, 70 mmol). The reaction was stirred at 0°C for 2 h. At RT the DCM layer was separated and washed with sat. sodium bicarbonate solution, filtered through a hydrophobic filter and concentrated under reduced pressure to give the title compound (12.97 g, 100 % yield) which was used directly in the next step.
1H-NMR (400MHz, DMSO-de): d [ppm]= 7.51 (dd, 1 H), 7.35 (dd, 1 H), 7.20 (t, 1 H), 3.85 -3.91 (m, 3H).
Intermediate 4-1
tert- butyl 5-[(3-chloro-2-methoxyphenyl)carbamothioyl]-4-hydroxy-6-oxo-3,6-dihydropyridine-1(2/-/)-carboxylate
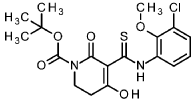
To an ice-cooled solution of 1-chloro-3-isothiocyanato-2-methoxybenzene (intermediate 3-1 , 4.00 g, 20.0 mmol) and tert- butyl 2,4-dioxopiperidine-1-carboxylate (CAS 845267-78-9, 4.27 g, 20.0 mmol) in acetonitrile (92 ml) was added dropwise DBU (4.5 ml, 30 mmol). The reaction was stirred at RT overnight. To the reaction mixture was added ice-water (200 ml_) and cone. HCI (2 ml_). The mixture was stirred for 20 min. and extracted with DCM. The organic phase was filtered over a water-repellent filter, conentrated under reduced pressure and purified by flash chromatography (silica, hexane / EtOAc gradient 0-50 %) to give 6.54 g of the title compound (71 % yield).
1H-NMR (400MHz, DMSO-de): d [ppm]= 13.36 (br s, 1 H), 7.73 (d, 1 H), 7.47 (dd, 1 H), 7.22 (t, 1 H), 3.76 – 3.82 (m, 5H), 2.88 (t, 2H), 1.48 (s, 9H).
LC-MS (method 1): Rt = 1.49 min; MS (ESIpos): m/z = 413.1 [M+H]+
Intermediate 5-1
A/-(3-chloro-2-methoxyphenyl)-4-hydroxy-2-oxo-1 ,2,5,6-tetrahydropyridine-3-carbothioamide
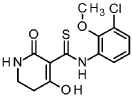
To a solution of tert- butyl 5-[(3-chloro-2-methoxyphenyl)carbamothioyl]-4-hydroxy-6-oxo-3,6-dihydropyridine-1 (2/-/)-carboxylate (intermediate 4-1 , 6.54 g, 15.8 mmol) in dichloromethane (94 ml) was added TFA (12 ml, 160 mmol) and the mixture was stirred 1.5 h at RT. The reaction mixture was concentrated under reduced pressure and the residue was solved in EtOAc and washed with sat. sodium bicarbonate solution and brine. The organic layer was filtered through a hydrophobic filter and the filtrate was dried to dryness. The residue was purified by flash chromatography (silica, hexane / EtOAc gradient 20-100 %) to give 4.06 g of the title compound (78 % yield).
1H-NMR (400 MHz, DMSO-de): d [ppm]= 16.45 (d, 1 H), 14.69 (s, 1 H), 14.33 (s, 1 H), 9.37 (br s, 1 H), 8.18 (br s, 1 H), 7.76 – 7.87 (m, 1 H), 7.37 – 7.45 (m, 1 H), 7.15 – 7.23 (m, 1 H), 3.73 – 3.76 (m, 3H), 3.43 (td, 1 H), 3.27 – 3.32 (m, 1 H), 2.79 (t, 1 H), 2.59 – 2.69 (m, 1 H).
LC-MS (method 1): Rt = 1.19 min; MS (ESIpos): m/z = 313 [M+H]+
ntermediate 6-2
A/-(3-chloro-2-methoxyphenyl)-4-{[(3-{[(2S)-1 ,4-dioxan-2-yl]methoxy}pyridin-4-yl)methyl]amino}-2-oxo-1 ,2,5,6-tetrahydropyridine-3-carbothioamide
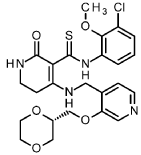
A mixture of A/-(3-chloro-2-methoxyphenyl)-4-hydroxy-2-oxo-1 ,2,5,6-tetrahydropyridine-3-carbothioa ide (intermediate 5-1 , 866 mg, 2.77 mmol) and 1-(3-{[(2S)-1 ,4-dioxan-2-yl]methoxy}pyridin-4-yl)methanamine (intermediate 2-8, 776 mg, 80% purity, 2.77 mmol) in ACN (22 ml) was treated with A/,0-bis(trimethylsilyl)acetamide (2.05 ml, 8.6 mmol, CAS 10416-59-8) and stirred at 80°C for 4 h. The reaction mixture was concentrated under reduced pressure and purified by flash chromatography (silica, DCM / EtOH gradient 0-20%) to give 1.23 g (95% purity, 81 % yield) of the title compound.
1H-NMR (400MHz, DMSO-d6): d [ppm]= 2.78 (t, 2H), 3.16 (td, 2H), 3.40 – 3.54 (m, 3H), 3.59 – 3.69 (m, 2H), 3.71 (s, 3H), 3.73 – 3.79 (m, 1 H), 3.83 – 3.95 (m, 2H), 4.16 (t, 2H), 4.67 (d, 2H), 7.11 (t, 1 H), 7.27 – 7.33 (m, 2H), 7.73 (br s, 1 H), 7.81 (dd, 1 H), 8.24 (d, 1 H), 8.39 (s, 1 H), 13.69 (s, 1 H), 14.79 (s, 1 H).
LC-MS (method 2): Rt = 1.09 min; MS (ESIpos): m/z = 519 [M+H]+
Example 2
3-(3-chloro-2-methoxyanilino)-2-(3-{[(2S)-1 ,4-dioxan-2-yl]methoxy}pyridin-4-yl)-1 ,5,6,7-tetrahydro-4H-pyrrolo[3,2-c]pyridin-4-one (Stereoisomer 1)

The title compound from example 1 (140 mg) was separated into enantiomers by preparative chiral HPLC to give title compound (enantiomer 1 , 27 mg at Rt = 14.0 – 17.0 min) and enantiomer 2 (25 mg at Rt = 20.0 – 24.8 min, see example 3).
Preparative chiral HPLC method:
Instrument: Labomatic HD5000, Labocord-5000; Gilson GX-241 , Labcol Vario 4000; column: Cellulose SB 5m, 250×30 mm; eluent A: hexane + 0.1 vol. % diethylamine (99 %); eluent B: 2-propanol; isocratic: 50 % A + 50 % B; flow 50 ml/min; UV: 254 nm.
Analytical chiral HPLC method:
Instrument: Agilent HPLC 1260; column: Cellulose SB 3m, 100×4.6 mm; eluent A: hexane + 0.1 vol. % diethylamine (99 %); eluent B: 2-propanol; isocratic: 50 % A + 50 % B, flow 1.4 ml/min; temperature: 25°C; UV: 254 nm
Analytical chiral HPLC: Rt = 4.49 min.
Optical rotation:[a]D = 1.7° +/- 0.98° (c = 3.6 mg/2 ml, methanol)
Enantioselective synthesis confirmed the title compound as 3-(3-chloro-2-methoxyanilino)-2-(3-{[(2S)-1 ,4-dioxan-2-yl]methoxy}pyridin-4-yl)-1 ,5,6,7-tetrahydro-4/-/-pyrrolo[3,2-c]pyridin-4-one. 872 mg (95% purity, 72% yield) of the title compound were prepared in analogy to example 1 using A/-(3-chloro-2-methoxyphenyl)-4-{[(3-{[(2S)-1 ,4-dioxan-2-yl]methoxy}pyridin-4-yl)methyl]amino}-2-oxo-1 ,2,5,6-tetrahydropyridine-3-carbothioamide (intermediate 6-2, 1.23 g, 2.36 mmol) as starting material, followed by purification with preparative HPLC (method 10, gradient: 0.00-0.50 min 15% B, 0.50-6.00 min 15-55% B).
1H-NMR (400MHz, DMSO-d6): d [ppm]= 2.86 (t, 2H), 3.38 – 3.47 (m, 3H), 3.53 (td, 1 H), 3.69
– 3.78 (m, 2H), 3.83 (dd, 1 H), 3.88 (s, 3H), 3.90 (m, 1 H), 3.98 – 4.08 (m, 1 H), 4.12 – 4.18 (m, 1 H), 4.28 (dd, 1 H), 6.12 – 6.17 (quin, 1 H), 6.66 – 6.71 (m, 2H), 7.16 (s, 1 H), 7.28 (d, 1 H),
7.52 (s, 1 H), 8.04 (d, 1 H), 8.39 (s, 1 H), 11.07 (s, 1 H).
Analytical chiral HPLC: Rt = 4.46 min.
Optical rotation:[a]D = -12.5° +/- 0.52° (c = 5.6 mg/ l, chloroform)
PAT
- 4H-pyrrolo[3,2-c]pyridin-4-one compoundPublication Number: CN-114127064-BPriority Date: 2019-04-24Grant Date: 2023-12-26
- 4H-Pyrrolo[3,2-c]pyridin-4-one compoundsPublication Number: CN-117946100-APriority Date: 2019-04-24
- 4H-pyrrolo [3,2-c ] pyridin-4-one compoundsPublication Number: CN-117986251-APriority Date: 2019-04-24
- 4h-pyrrolo[3,2-c]pyridin-4-one compoundsPublication Number: TW-I849114-BPriority Date: 2019-04-24Grant Date: 2024-07-21
- 4H-pyrrolo[3,2-c]pyridin-4-one compoundPublication Number: KR-20220004103-APriority Date: 2019-04-24
- 4H-PYRROLO[3,2-C]PYRIDIN-4-ONE COMPOUNDSPublication Number: PE-20220254-A1Priority Date: 2019-04-24
- 4H-Pyrrolo [3,2-C] Pyridine-4-one CompoundPublication Number: JP-2022532850-APriority Date: 2019-04-24
- 4h-pyrrolo[3,2-c]pyridin-4-one compoundsPublication Number: US-2022298157-A1Priority Date: 2019-04-24
- 4H-PYRROL[3,2-C]PYRIDIN-4-ONE, ITS USES, PHARMACEUTICAL COMPOSITION, AND KIT OF PARTSPublication Number: BR-112021019998-B1Priority Date: 2019-04-24
- 4h-pyrrolo[3,2-c]pyridin-4-one compoundsPublication Number: WO-2020216781-A1Priority Date: 2019-04-24
- 4h-pyrrolo[3,2-c]pyridin-4-one compoundsPublication Number: TW-202106683-APriority Date: 2019-04-24
- 4H-pyrrolo(3,2-c)pyridin-4-one compoundsPublication Number: AU-2020262221-A1Priority Date: 2019-04-24
- 4H-pyrrolo [3,2-c ] pyridin-4-one compoundsPublication Number: CN-114127064-APriority Date: 2019-04-24
- 4h-pyrrolo[3,2-c]pyridin-4-one compoundsPublication Number: EP-3959211-A1Priority Date: 2019-04-24



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
Medical uses
Sevabertinib is indicated for the treatment of adults with locally advanced or metastatic non-squamous non-small cell lung cancer whose tumors have HER2 (ERBB2) tyrosine kinase domain activating mutations.[1][2]
Adverse effects
The US prescribing information includes warnings and precautions for diarrhea, hepatotoxicity, interstitial lung disease/pneumonitis, ocular toxicity, pancreatic enzyme elevation, and embryo-fetal toxicity.[2]
History
Efficacy was evaluated in people with unresectable or metastatic, non-squamous non-small cell lung cancer with HER2 (ERBB2) tyrosine kinase domain activating mutations who had received prior systemic therapy and received sevabertinib in SOHO-01 (NCT05099172), an open-label, single-arm, multi-center, multi-cohort clinical trial.[2] HER2 (ERBB2) activating mutations were determined in tumor tissue or plasma by local laboratories prior to enrollment.[2]
The US Food and Drug Administration granted the application for sevabertinib priority review, breakthrough therapy, and orphan drug designations.[2]
Society and culture
Legal status
Sevabertinib was approved for medical use in the United States in November 2025.[3][4]
Names
Sevabertinib is the international nonproprietary name.[5]
Sevabertinib is sold under the brand name Hyrnuo.[1][3]
References
- “HYRNUO (sevabertinib) tablets, for oral use” (PDF). Bayer HealthCare Pharmaceuticals Inc. U.S. Food and Drug Administration.
- “FDA grants accelerated approval to sevabertinib for non-squamous non-small cell lung cancer”. U.S. Food and Drug Administration (FDA). 19 November 2025. Retrieved 21 November 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - “U.S. FDA Approves Hyrnuo (sevabertinib) for Previously Treated Patients with HER2-Mutated Locally Advanced or Metastatic Non-Squamous Non-Small Cell Lung Cancer” (Press release). Bayer. 20 November 2025. Retrieved 21 November 2025 – via Business Wire.
- “U.S. FDA grants accelerated approval to Bayer’s Hyrnuo (sevabertinib) for patients with previously treated advanced HER2-mutant non-small cell lung cancer”. Bayer (Press release). 20 November 2025. Retrieved 21 November 2025.
- World Health Organization (2025). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 93”. WHO Drug Information. 39 (1). hdl:10665/381075.
Further reading
- Le X, Kim TM, Loong HH, Prelaj A, Goh BC, Li L, et al. (November 2025). “Sevabertinib in Advanced HER2-Mutant Non-Small-Cell Lung Cancer”. The New England Journal of Medicine. 393 (18): 1819–1832. doi:10.1056/NEJMoa2511065. PMID 41104928.
- Siegel F, Siegel S, Kotýnková K, Karsli Uzunbas G, Korr D, Tomono H, et al. (October 2025). “Sevabertinib, a Reversible HER2 Inhibitor with Activity in Lung Cancer”. Cancer Discovery: OF1 – OF14. doi:10.1158/2159-8290.CD-25-0605. PMID 41090369.
External links
- “Sevabertinib”. NCI Drug Dictionary.
- “Sevabertinib ( Code – C185187 )”. EVS Explore.
- Clinical trial number NCT05099172 for “First in Human Study of BAY2927088 in Participants Who Have Advanced Non-small Cell Lung Cancer (NSCLC) With Mutations in the Genes of Epidermal Growth Factor Receptor (EGFR) and/or Human Epidermal Growth Factor Receptor 2 (HER2)” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Trade names | Hyrnuo |
| Other names | BAY2927088, sevabertinib hydrate (JAN JP) |
| License data | US DailyMed: Sevabertinib |
| Routes of administration | By mouth |
| Drug class | Antineoplastic |
| ATC code | None |
| Legal status | |
| Legal status | US: ℞-only[1] |
| Identifiers | |
| CAS Number | 2521285-05-0 |
| PubChem CID | 155234713 |
| DrugBank | DB21667 |
| ChemSpider | 129786615 |
| UNII | 2A7VPM5RWH |
| KEGG | D13098 |
| Chemical and physical data | |
| Formula | C24H25ClN4O5 |
| Molar mass | 484.94 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
/////sevabertinib, FDA 2025, APPROVALS 2025, Hyrnuo, 2A7VPM5RWH, BAY-2927088, BAY 2927088














