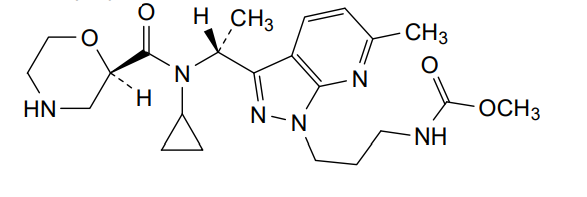
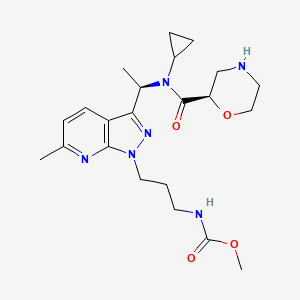
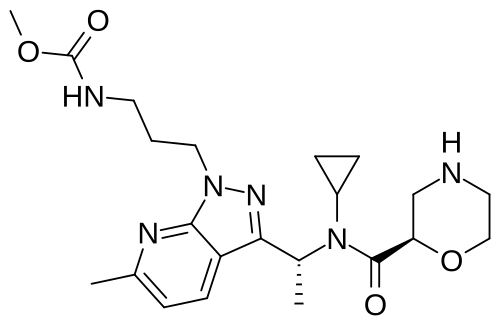
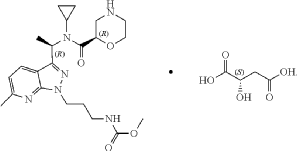
Sitokiren
CAS 1399849-02-5,
MF C22H32N6O4, 444.5 g/mol
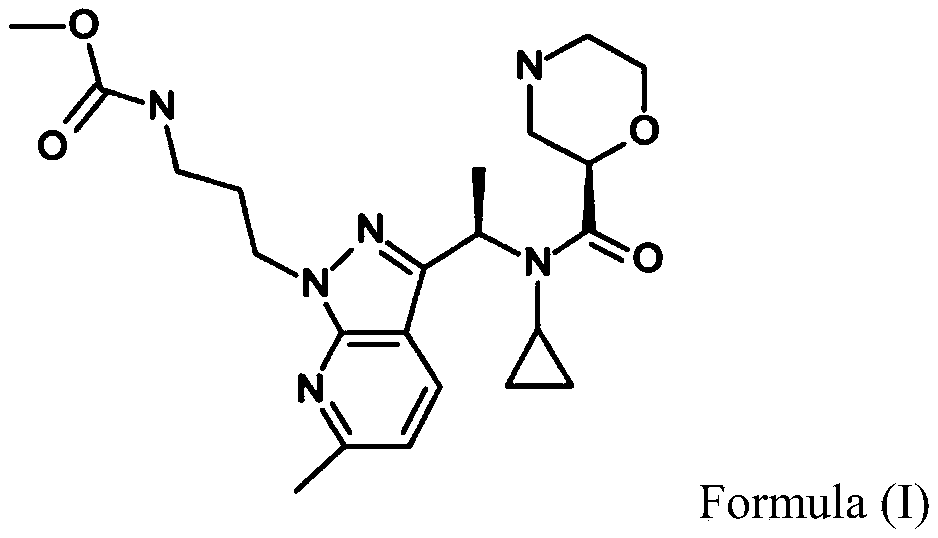
methyl N-[3-[3-[(1R)-1-[cyclopropyl-[(2R)-morpholine-2-carbonyl]amino]ethyl]-6-methylpyrazolo[5,4-b]pyridin-1-yl]propyl]carbamate
methyl [3-(3-{(1R)-1-[(2R)-N-cyclopropylmorpholine-2-carboxamido]ethyl}-6-methyl-1H-pyrazolo[3,4-
b]pyridin-1-yl)propyl]carbamate
renin inhibitor, SPH 3127, C2M78A9V6Z
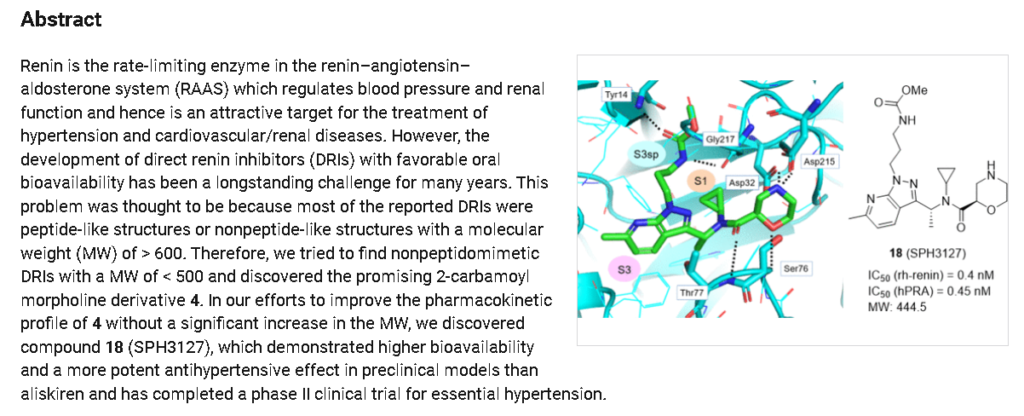
Sitokiren, also known as SPH3127, isa highly potent, orally active direct renin inhibitor developed by Mitsubishi Tanabe Pharma Corp. that was initially investigated for hypertension and cardiovascular diseases. Recent research has shown it also has a strong anti-inflammatory effect, particularly in the gut, making it a potential candidate for treating conditions like inflammatory bowel disease (IBD).
What it is
- Direct renin inhibitor: Sitokiren directly inhibits the enzyme renin, which is the rate-limiting step in the renin-angiotensin-aldosterone system (RAAS).
- Chemical properties: It is a small molecule with the chemical formula
C22H32N6O4
- Developed by: Mitsubishi Tanabe Pharma Corp..
- Alternative name: SPH3127 is another name for sitokiren.
How it works
- Blocks the RAAS: By inhibiting renin, it prevents the RAAS from over-activating.
- Potential benefits: This inhibition may help in managing blood pressure and has also shown promise in suppressing inflammation in the gut, which is a key factor in IBD.
Current research and potential applications
- Hypertension: Sitokiren was initially developed for its potential to treat hypertension, and preclinical models have shown it to be more potent than the approved drug aliskiren.
- Inflammatory bowel disease (IBD): Studies using sitokiren in mouse models have demonstrated its ability to reduce inflammation and protect against damage in colitis, suggesting it could be a novel therapeutic for IBD.
SPH-3127 is under investigation in clinical trial NCT05359068 (Study to Evaluate the Efficacy and Safety of SPH3127 in Patients With Mild-moderate Essential Hypertension).
SPH3127 is a small-molecule renin inhibitor developed by Shanghai Pharmaceuticals for hypertension and kidney disease. It is believed to be more potent than aliskiren.[1][2][3]
SYN
https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.2c00834
Methyl N-[3-(3-{(1S)-1-[cyclopropyl-((2R)-morpholine-2-carbonyl)amino]ethyl}-6-methyl
pyrazolo[3,4-b]pyridin-1-yl)propyl]carbamate (18-diastereomer). This isomer was separated
from a mixture of the corresponding diastereomers using NH-silica gel column chromatography
as the more polar isomer. 1H NMR (400 MHz, DMSO-d6) : 0.20 (m, 1H), 0.51−0.74 (m, 3H),
1.70 (d, J = 7.0 Hz, 3H), 1.94 (m, 2H), 2.57 (s, 3H), 2.60−2.75 (m, 3H), 2.79 (m, 1H), 2.87 (dd,J = 2.4, 12.5 Hz, 1H), 3.00 (m, 2H), 3.47 (m, 1H), 3.51 (s, 3H), 3.79 (d, J = 10.9 Hz, 1H),
4.30−4.46 (m, 2H), 4.66 (dd, J = 2.1, 9.4 Hz, 1H), 5.84 (q, J = 7.0 Hz, 1H), 7.02 (d, J = 8.2 Hz,
1H), 7.16 (m, 1H), 7.83 (d, J = 8.2 Hz, 1H). MS (APCI) m/z: 445.1 [M + H]+. Purity and
diastereomeric excess measured by chiral HPLC: 98.37%, 81.23% de (column: Chiralpak IC (4.6
mm × 250 mm, elution: hexane/EtOH/diethylamine, 50:50:0.1 (v/v), flow rate: 0.5 mL/min,
column temperature: 25 °C, retention time: 29.40 min).
SYN
SYN
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022047730&_cid=P12-MIR63I-81994-1
PAT
Nitrogen-containing saturated heterocyclic compound
Publication Number: US-9278944-B2
Priority Date: 2011-03-16
Grant Date: 2016-03-08
https://patentscope.wipo.int/search/en/detail.jsf?docId=US95781978&_cid=P12-MIR64F-82901-1
PAT
- Salt of morpholine derivative and crystalline form thereof, as well as preparation method, pharmaceutical composition and use of the samePublication Number: EP-3398946-B1Priority Date: 2015-12-29Grant Date: 2022-05-04
- Nitrogen-containing saturated heterocyclic compoundPublication Number: EP-2687518-B1Priority Date: 2011-03-16Grant Date: 2017-11-01
- Nitrogen-containing saturated heterocyclic compoundPublication Number: US-10155731-B2Priority Date: 2011-03-16Grant Date: 2018-12-18
- Nitrogen-containing saturated heterocyclic compoundPublication Number: US-2014011807-A1Priority Date: 2011-03-16
- Nitrogen-containing saturated heterocyclic compoundPublication Number: US-2016145220-A1Priority Date: 2011-03-16
- Methods to treat inflammatory bowel diseasePublication Number: US-2023398123-A1Priority Date: 2020-09-04
- Salt of morpholine derivative and crystalline form thereof, as well as preparation method, pharmaceutical composition and use of the samePublication Number: EP-3398946-A1Priority Date: 2015-12-29
- Salts of morpholine derivative, crystal forms thereof, processes for producing the same, pharmaceutical compositions including the same, and use thereofPublication Number: US-10519150-B2Priority Date: 2015-12-29Grant Date: 2019-12-31
- Salts of morpholine derivative, crystal forms thereof, processes for producing the same, pharmaceutical compositions including the same, and use thereofPublication Number: US-2019016718-A1Priority Date: 2015-12-29
- Salt of morpholine derivative and its crystal form, manufacturing method thereof, pharmaceutical composition and use thereofPublication Number: TW-I705065-BPriority Date: 2015-12-29Grant Date: 2020-09-21
- Methods to treat inflammatory bowel diseasePublication Number: WO-2022047730-A1Priority Date: 2020-09-04
- Application of nitrogen-containing saturated heterocyclic compoundPublication Number: WO-2022048614-A1Priority Date: 2020-09-04
- Methods to treat inflammatory bowel diseasePublication Number: WO-2022048618-A1Priority Date: 2020-09-04
- Application of nitrogen-containing saturated heterocyclic compoundPublication Number: EP-4209218-A1Priority Date: 2020-09-04
- Application of nitrogen-containing saturated heterocyclic compoundPublication Number: US-2023330093-A1Priority Date: 2020-09-04



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Iijima, Daisuke; Sugama, Hiroshi; Takahashi, Yoichi; Hirai, Miki; Togashi, Yuko; Xie, Jianshu; Shen, Jingkang; Ke, Ying; Akatsuka, Hidenori; Kawaguchi, Takayuki; Takedomi, Kei; Kashima, Akiko; Nishio, Masashi; Inui, Yosuke; Yoneda, Hikaru; Xia, Guangxin; Iijima, Toru (25 August 2022). “Discovery of SPH3127: A Novel, Highly Potent, and Orally Active Direct Renin Inhibitor”. Journal of Medicinal Chemistry. 65 (16): 10882–10897. doi:10.1021/acs.jmedchem.2c00834. PMID 35939295. S2CID 251400126.
- Zhang, Leduo; Mao, Yu; Gao, Zhiwei; Chen, Xiaoyan; Li, Xin; Liu, Yanjun; Xia, Guangxin (February 2020). “The Nonclinical Pharmacokinetics and Prediction of Human Pharmacokinetics of SPH3127, a Novel Direct Renin Inhibitor”. European Journal of Drug Metabolism and Pharmacokinetics. 45 (1): 15–26. doi:10.1007/s13318-019-00573-9. PMID 31494843. S2CID 201848935.
- Jing, Shan; Xu, Ranchi; Yang, Kexu; Liu, Wenfang; Zhang, Leduo; Ke, Ying; Xia, Guangxin; Lin, Yang (April 2021). “Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of SPH3127: A Phase I, Randomized, Double-Blind, Placebo-Controlled Trial”. Clinical Therapeutics. 43 (4): 735.e1–735.e14. doi:10.1016/j.clinthera.2021.01.025. PMID 33653620. S2CID 232104329.
| Legal status | |
|---|---|
| Legal status | Investigational |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1399849-02-5 |
| PubChem CID | 117877477 |
| ChemSpider | 76799450 |
| UNII | C2M78A9V6Z |
| ChEMBL | ChEMBL4110551 |
| Chemical and physical data | |
| Formula | C22H32N6O4 |
| Molar mass | 444.536 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
////Sitokiren, renin inhibitor, SPH 3127, C2M78A9V6Z














