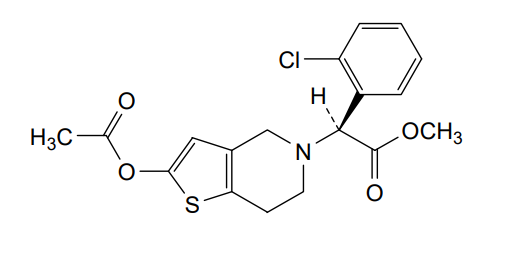
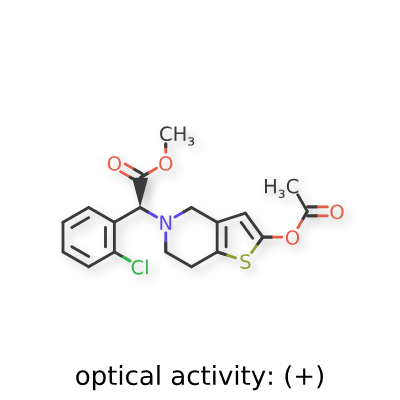
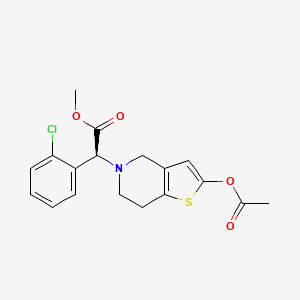
Sumecigrel, VICAGREL
CAS 1314081-53-2
MF C18H18ClNO4S MW379.858
- (S)-2-(2-ACETOXY-6,7-DIHYDROTHIENO(3,2-C)PYRIDINE-5(4H)-YL)-2-(2-CHLOROPHENYL)ACETIC ACID METHYL ESTER
- METHYL (.ALPHA.S)-2-(ACETYLOXY)-.ALPHA.-(2-CHLOROPHENYL)-6,7-DIHYDROTHIENO(3,2-C)PYRIDINE-5(4H)-ACETATE
- METHYL (2S)-2-(2-ACETYLOXY-6,7-DIHYDRO-4H-THIENO(3,2-C)PYRIDIN-5-YL)-2-(2-CHLOROPHENYL)ACETATE
- METHYL (S)-(2-(ACETYLOXY)-6,7-DIHYDROTHIENO(3,2-C)PYRIDIN-5(4H)-YL)(2-CHLOROPHENYL)ACETATE
- THIENO(3,2-C)PYRIDINE-5(4H)-ACETIC ACID, 2-(ACETYLOXY)-.ALPHA.-(2-CHLOROPHENYL)-6,7-DIHYDRO-, METHYL ESTER, (.ALPHA.S)-
- VICAGREL
methyl (S)-2-(acetyloxy)-6,7-dihydrothieno[3,2- c]pyridin-5(4H)-ylacetate
platelet aggregation inhibitor, 8A63K3TN0U, VICAGREL
- Pharmacokinetic/Pharmacodynamic Study of Vicagrel Capsules and Clopidogrel Tablets in Healthy CYP2C19 Normal MetabolizersCTID: NCT07067775Phase: Phase 1Status: CompletedDate: 2025-09-09
- Efficacy and Safety Study of Vicagrel in Patients With Acute Coronary Syndrome (ACS) Undergoing Percutaneous Coronary Intervention (PCI)CTID: NCT06577519Phase: Phase 3Status: RecruitingDate: 2024-10-01
- PK/PD Study of Vicagrel and Clopidogrel in Healthy Subjects With Different CYP2C19 MetabolizersCTID: NCT05162053Phase: Phase 1Status: CompletedDate: 2023-11-03
- A Clinical Trial to Evaluate the Effect of Food on PK and PD of Vicagrel Capsules in Healthy Adult SubjectsCTID: NCT04919551Phase: Phase 1Status: CompletedDate: 2021-11-01
- The Efficacy, Safety and Pharmacokinetic of Antiplatelet Therapy for VicagrelCTID: NCT03599284Phase: Phase 2Status: CompletedDate: 2019-09-23
- Pharmacokinetics and Pharmacodynamics of Vicagrel in Healthy Adult Subjects of Different CYP2C19
- CTID: NCT03942458
- Phase: Phase 1
- Status: Completed
- Date: 2019-09-19
Sumecigrel (also known as
vicagrel) is an investigational small molecule drug classified as a P2Y12 inhibitor and antiplatelet agent. It is currently under clinical development for the treatment of various cardiovascular and peripheral conditions.
Key Information
- Therapeutic Class: Antiplatelet agent; P2Y12 inhibitor. These types of drugs work by preventing platelets in the blood from sticking together and forming clots, which is a key process in conditions like heart attack and stroke.
- Developer: Jiangsu Vcare PharmaTech.
- Clinical Status: It is currently in the pre-registration phase for acute coronary syndrome (ACS). It has also been under investigation for ischemic stroke and peripheral arterial disease.
- Synonyms: The drug is also widely referred to by its USAN (United States Adopted Name) and INN (International Nonproprietary Name) designation, vicagrel.
Chemical Details
- Formula:
C18H18ClNO4SC sub 18 H sub 18 ClNO sub 4 SC18H18ClNO4S.
- CAS Number: 1314081-53-2.
- UNII: 8A63K3TN0U.
For more detailed information regarding its regulatory status, you can check the official precisionFDA or PubChem databases.
SYN
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN85433897&_cid=P22-MITPA1-71386-1
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=EP75374721&_cid=P22-MITPA1-71386-1
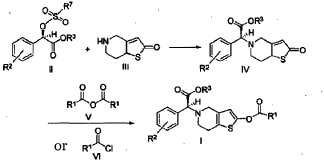
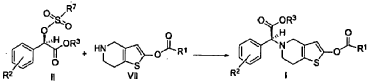
Example 3
(2S)-Methyl 2-(2-oxo-7,7a-dihydrothieno[3,2-c]pyridin-5(2H,4H,6H)-yl)-2-(2-chlorophenyl)-acetate (IV-1)
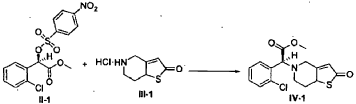
[0036] 58.1 g (0.15 mol) of (R)-methyl 2-(2-chlorophenyl)-2-(4-nitrophenylsulfonyloxy)-acetate ( II-1), 32.3 g (0.17 mol) of 5,6,7,7a-tetrahydrothieno[3,2-c]pyridin-2(4H)-one hydrochloride ( III-1), and 37.8g (0.38 mol) of potassium bicarbonate were added to 500 ml of acetonitrile. The reaction was stirred under a nitrogen atmosphere at room temperature for 26 hrs. The reaction solution was allowed to stand and the insoluble material was filtered off, to obtain a dark red mother liquor. The solvent was evaporated under reduced pressure, and 35.4 g of an oil product was obtained after purification by flash column chromatography (petroleum ether:ethyl acetate = 4:1). Yield 70%. Recrystalization from ethanol afforded 18.1 g of a pure product (IV-1) as a white solid. mp: 146-148°C, ee = 97.5%, [α] D 19 = +114.0° (c 0.5, MeOH); 1H-NMR (300 MHz, CDCl 3) δ 1.79-1.93 (m, 1 H), 2.30-2.40 (m, 1 H), 2.56-2.70 (m, 1 H), 3.00-3.27 (m, 2 H), 3.72 (s, 3 H), 3.79-3.93 (m, 1 H), 4.12-4.19 (m, 1 H), 4.89 (d, 1 H, J= 5.6 Hz), 6.00 (d, 1 H, J = 5.2 Hz), 7.26-7.50 (m, 4 H); 13C-NMR (75 MHz, CDCl 3) δ 33.9, 34.0, 49.0, 49.7, 51.1, 51.6, 52.2, 52.4, 67.3, 76.6, 77.0, 77.4, 126.6, 126.8, 127.2, 129.8, 130.1, 132.7, 134.8, 167.2, 167.4, 170.8, 198.6; ESI-MS m/ z 338.1 [M+H] +; HRMS Calcd for C 16H 17NO 3SCl [M+H] + m/ z 338.0618, found 338.0626.
Reference Example 4
(S)-Methyl 2-(2-benzoyloxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate (I-1)
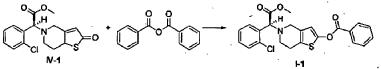
[0038] (2S)-Methyl 2-(2-chlorophenyl)-2-(2-oxo-5,6,7,7a-tetrahydrothieno[3,2-c]pyridinyl)acetate ( IV– 1) (113 mg) was dissolved in acetonitrile (10 ml), 0.10 ml of triethylamine was added, and 151 mg of benzoic anhydride was added dropwise at 0°C, and then the mixture was warmed to room temperature and reacted for 2 hrs. The reaction solution was poured into water (30 ml), the aqueous phase was extracted with ethyl acetate (50 ml x 3), and the organic phase was washed with saturated aqueous sodium bicarbonate solution and saturated saline, dried over anhydrous sodium sulfate, and evaporated, to obtain a crude product, which was subjected to flash column chromatography (petroleum ether:ethyl acetate = 40 : 3), to obtain (S)-methyl 2-(2-benzoyloxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate (I-1) (77 mg). Yield 52%, mp: 84-86°C, ee = 93.5% (chiral HPLC analysis conditions: Chiralpak IC 4.6 mm x 250 mm; column temperature: 25° C; mobile phase: 90% n-hexane/10% isopropanol/0.1% diethylamine; flow rate: 0.5 ml/min; and detection wavelength: UV 254 nm), [α] D20 = +34.00° (c 0.50, MeOH); 1H-NMR (300 MHz, CDCl 3) δ 2.82-2.93 (m, 4 H), 3.57-3.68 (m, 2 H), 3.73 (s, 3 H), 4.95 (s, 1 H), 6.42 (s, 1 H), 7.26-8.17 (m, 9 H); 13C-NMR (75 MHz CDCl 3) δ 25.0, 48.2, 50.4, 52.2, 67.8, 112.1, 125.9, 127.2, 128.5, 128.6, 129.5, 129.8, 130.0, 130.2, 133.9, 134,7, 149.9, 163.5; ESI-MS m/ z 442.1 [M+H] +; HRMS Calcd for C 23H 21NO 4SCl [M+H] +m/ z 442.0891, found 442.0880.
Example 5
(S)-Methyl 2-(2-acetoxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate (I-2)
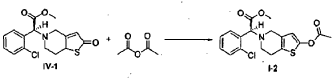
[0040] Following the method described in Example 4, (2S)-methyl 2-(2-chlorophenyl)-2-(2-oxo-5, 6, 7, 7a-tetrahydrothieno[3,2-c]pyridinyl)acetate (IV-1) (6.5 g) was reacted with acetic anhydride (3.6 ml), to prepare (S)-methyl 2-(2-acetoxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate ( I-2) (6.8 g). Yield 93%. Recrystallization from ethanol afforded a white solid, mp: 73-75°C, ee = 98.9% (chiral HPLC analysis conditions: Chiralpak IC 4.6 mm x 250 mm; column temperature: 25°C; mobile phase: 92% n-hexane/8% tetrahydrofuran/0.1% diethylamine; flow rate: 0.5 ml/min; and detection wavelength: UV 254 nm), [α] D23 = +45.00°(c = 1.0, CH 3OH); 1H-NMR (300 MHz, CDCl 3) δ 2.26 (s, 3 H), 2.65-2.90 (m, 4 H), 3.47-3.69 (m, 2 H), 3.72 (s, 3 H), 4.92 (s, 1 H), 6.26 (s, 1 H), 7.24-7.70 (m, 4 H); 13C-NMR (75 MHz, CDCl 3) δ 20.2, 24.5, 47.6, 49.8, 51.6, 67.3, 111.5, 125.3, 126.6, 128.8, 128.9, 129.3, 129.4, 133.3, 134.2, 149.1, 167.2, 170.7; ESI-MS m/ z 380.0 [M+H] +; HRMS Calcd for C 18H 19NO 4SCl [M+H] +m/ z 380.0723, found 380.0737.
Reference Example 6
(R)-Methyl 2-(2-acetoxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate (I-2′)
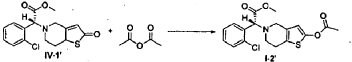
[0042] Following the method described in Example 4, (2R)-methyl 2-(2-chlorophenyl)-2-(2-oxo-7,7a-dihydrothieno[3.2-c]pyridin-5(2H,4H,6H)-yl)-acetate ( IV-1′) (prepared following Examples 1-3) was reacted with acetic anhydride, to prepare (R)-methyl 2-(2-acetoxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate ( I-2′), ee = 98.2% (chiral HPLC analysis conditions were the same as those in Example 5), [α] D 23 =-44.00° (c= 1.0, CH 3OH).
Reference Example 7
(S)-Methyl 2-(2-propanoyloxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate (I-3)
[0043] Following the method described in Example 4, (2S)-methyl 2-(2-chlorophenyl)-2-(2-oxo-7, 7a-dihydrothieno[3.2-c]pyridin-5(2H, 4H,6H)-yl)-acetate ( IV-1) (338 mg) was reacted with propionic anhydride (0.27 ml), to prepare (S)-methyl 2-(2-propanoyloxy-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)-2-(2-chlorophenyl)-acetate ( I-3) (267 mg).Yield 66%, ee = 96.5% (chiral HPLC analysis conditions were the same as those in Example 4), [α] D20 = + 36.00°( c 0.50, MeOH); 1H-NMR (300 MHz, CDCl 3) δ 1.23 (t, 3 H, J = 7.4 Hz), 2.55 (q, 2 H, J= 7.7 Hz), 2.76-2.78 (m, 2 H), 2.87-2.88 (m, 2 H), 3.53 (d, 1 H, J = 14.2 Hz), 3.65 (d, 1 H, J = 13.6 Hz), 3.72 (s, 3 H), 4.91 (s, 1 H), 6.26 (s, 1 H), 7.26-7.69 (m, 4 H); 13C-NMR (75 MHz, CDCl 3) δ 8.8, 21.1, 25.0, 27.4, 48.2, 50.3, 52.2, 67.8, 106.2, 111.7, 125.6, 127.2, 129.1, 129.5, 129.8, 130.0, 123.7, 149.8, 171.2; ESI-MS m/ z 394.1 [M+H] +; HRMS Calcd for C 19H 21NO 4SCl [M+H] +m/ z 394.0883, found 394.0880.
PAT
- Optically active 2-hydroxytetrahydrothienopyridine derivatives, preparation method and use in manufacture of medicament thereofPublication Number: KR-102215429-B1Priority Date: 2010-02-02Grant Date: 2021-02-16
- Optically active 2-hydroxy tetrahydrothienopyridine derivatives, preparation method and use in manufacture of medicament thereofPublication Number: EP-3290423-B1Priority Date: 2010-02-02Grant Date: 2021-07-21
- Optically active 2-hydroxy tetrahydrothienopyridine derivatives, preparation method and use in manufacture of medicament thereofPublication Number: US-2015011583-A1Priority Date: 2010-02-02
- Optically active 2-hydroxy tetrahydrothienopyridine derivatives, preparation method and use in manufacture of medicament thereofPublication Number: US-2017121341-A1Priority Date: 2010-02-02
- Optically active 2-hydroxy tetrahydrothienopyridine derivatives, preparation method and use in manufacture of medicament thereofPublication Number: US-2019055260-A1Priority Date: 2010-02-02
- Optically active 2-hydroxy tetrahydrothienopyridine derivatives, preparation method and use in manufacture of medicament thereofPublication Number: US-8772489-B2Priority Date: 2010-02-02Grant Date: 2014-07-08
- Optically active 2-hydroxy tetrahydrothienopyridine derivatives, preparation method and use in manufacture of medicament thereofPublication Number: WO-2011095049-A1Priority Date: 2010-02-02


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
///////////Sumecigrel, platelet aggregation inhibitor, 8A63K3TN0U, VICAGREL














