
Surlorian
CAS 1467605-57-7
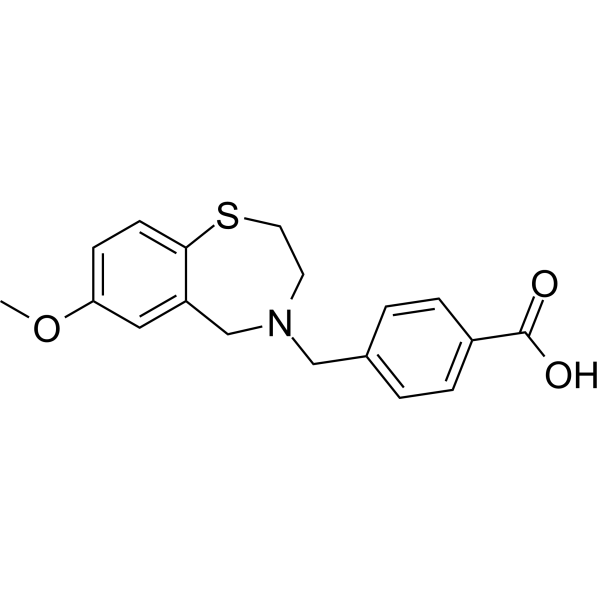
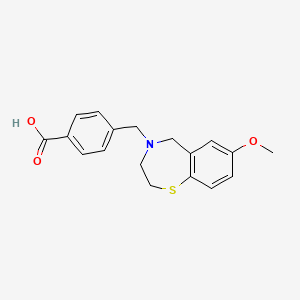
MFC18H19NO3S, 329.41
4-[(7-methoxy-2,3-dihydro-1,4-benzothiazepin-4(5H)-yl)methyl]benzoic acid
ryanodine receptor (RyR) stabilizer, ARM 210, RYCAL DMD, s48168, S 48168, 1033GN605L
Surlorian (ARM210) is a novel drug candidate, specifically a Ryanodine Receptor (RyR) stabilizer developed by RyCarma Therapeutics, designed to treat heart failure by repairing leaky RyRs in heart and skeletal muscles, aiming to improve both cardiac function and muscle weakness by restoring normal calcium regulation. It’s an allosteric modulator, a “Rycal,” that fixes these crucial calcium channels without blocking them, addressing a core issue in heart failure and related muscle diseases.
Key Aspects:
- Mechanism: It stabilizes damaged RyR channels, which become leaky due to stress (like in heart failure), preventing unregulated calcium leakage from the sarcoplasmic reticulum (SR).
- Target: Acts systemically on RyRs in both cardiac (heart) and skeletal (muscle) cells.
- Benefits: Aims to improve heart contraction/relaxation and alleviate skeletal muscle weakness, a common heart failure symptom.
- Therapeutic Area: Heart failure, potentially addressing underlying calcium dysregulation.
- Status: In clinical development, with Phase II trials underway as of mid-2025, according to AdisInsight.
- Chemical Info: Molecular Formula: C18H19NO3S; CAS No: 1467605-57-7.
In essence, Surlorian offers a new approach to heart failure by fixing the fundamental calcium handling problem in muscles, rather than just managing symptoms.
- OriginatorARMGO Pharma
- DeveloperNational Institute of Neurological Disorders and Stroke; RyCarma Therapeutics; Servier
- ClassAntiarrhythmics; Heart failure therapies; Small molecules
- Mechanism of ActionRyanodine receptor calcium release channel modulators
- Orphan Drug StatusYes – Polymorphic catecholergic ventricular tachycardia; Congenital structural myopathies; Duchenne muscular dystrophy
- Phase IIPolymorphic catecholergic ventricular tachycardia
- Phase ICardiac-arrhythmias; Congenital structural myopathies; Heart failure
- No development reportedDuchenne muscular dystrophy; Limb girdle muscular dystrophies; Sarcopenia; X-linked bulbo-spinal atrophy
- 04 Sep 2025Chemical structure information added.
- 02 Apr 2025Surlorian is still in phase I trial for Congenital structural myopathies in USA (PO) (NCT04141670)
- 02 Apr 2025Phase-I clinical trials in Heart failure (unspecified route), prior to April 2025 (RyCarma Therapeutics pipeline, April 2025)
- Treatment of an Inherited Ventricular ArrhythmiaCTID: NCT05122975Phase: Phase 2Status: TerminatedDate: 2024-09-19
- S 48168 (ARM 210) for the Treatment of RYR1-related Myopathies (RYR1-RM)CTID: NCT04141670Phase: Phase 1Status: CompletedDate: 2024-08-22
SYN
EXAMPLE 1: PREPARATION OF 4-[(7-METHOXY-2,3-DIHYDRO-1,4-BENZOTHIAZEPIN-4(5H)YL)METHYL]BENZOIC ACID
| 4-[(7-methoxy-2,3-dihydro-1,4-benzothiazepin-4(5H)yl)methyl]benzoic acid was prepared as described below. |
Stage 1: 7-methoxy-2,3,4,5-tetrahydrobenzo[f][1,4]thiazepine (“Amine”)

2-(4-Methoxyphenylthio)ethanamine (1)
Benzyl 2-(4-methoxyphenylthio)ethylcarbamate (2)
Benzyl 7-methoxy-2,3-dihydrobenzo[f][1,4]thiazepine-4(5H)-carboxylate (3)
7-Methoxy-2,3,4,5-tetrahydrobenzo[f][1,4]thiazepine hydrobromide (Amine)
Stage 2: −[(7-methoxy-2,3-dihydro-1,4-benzothiazepin-4(5H)yl)methyl]benzoic acid

In Scheme 2, L is a leaving group, which is, by way of example, a halogen or a sulfonate (OSO 2R′ wherein R′is alkyl or aryl, e.g., OMs (mesylate) or OTs (tosylate)). Amine (4) (1 mmol) was dissolved dichloromethane. To the solution was added alkylation reagent (5) (1 mmol), followed by N,N-diisopropylethylamine (2 mmol). The mixture was stirred at room temperature overnight. The solution was loaded onto a silica gel column directly and eluted with hexane/EtOAc (2:1, v/v) to afforded the desired product.
SYN
PAT
- Agents for treating disorders involving modulation of ryanodine receptorsPublication Number: US-2014088171-A1Priority Date: 2012-04-18
- Agents for treating disorders involving modulation of ryanodine receptorsPublication Number: US-2014378437-A1Priority Date: 2012-04-18
- Agents for treating disorders involving modulation of ryanodine receptorsPublication Number: US-8853198-B2Priority Date: 2012-04-18Grant Date: 2014-10-07
- Agents for treating disorders involving modulation of ryanodine receptorsPublication Number: WO-2013156505-A1Priority Date: 2012-04-18
- Drugs for treating diseases involved in the modulation of ryanodine receptorsPublication Number: JP-2015514736-APriority Date: 2012-04-18
- Drugs for treating diseases involved in the modulation of ryanodine receptorsPublication Number: JP-5965542-B2Priority Date: 2012-04-18Grant Date: 2016-08-10
- Agents for treating disorders involving modulation of ryanodine receptorsPublication Number: KR-101731459-B1Priority Date: 2012-04-18Grant Date: 2017-04-28
- Agents for treating disorders involving modulation of ryanodine receptorsPublication Number: KR-20150003347-APriority Date: 2012-04-18
- Agents for treating disorders involving modulation of ryanodine receptorsPublication Number: US-2013281512-A1Priority Date: 2012-04-18

- ARM-210 hemifumarate
- ZHR6WM1ADJ
- UNII-ZHR6WM1ADJ
- 1467606-11-6
- Surlorian fumarate (USAN)



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
///////////Surlorian, ryanodine receptor (RyR) stabilizer, ARM 210, RYCAL DMD, s48168, S 48168, 1033GN605L














