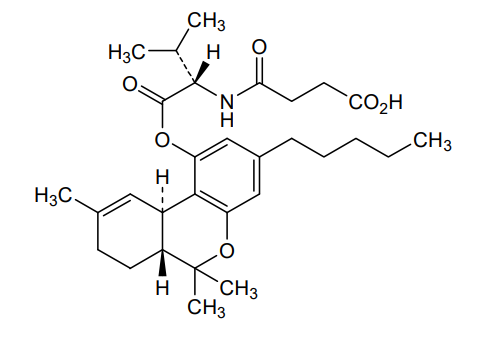
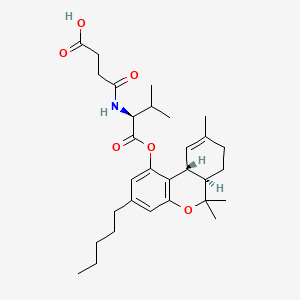
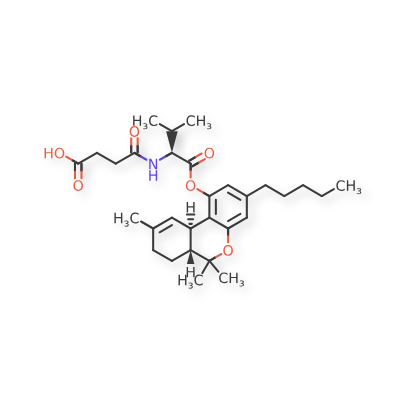
Suvadronabinol
CAS 1225194-84-2
MF C30H43NO6 MW513.7 g/mol
4-{[(2S)-3-methyl-1-oxo-1-{[(6aR,10aR)-6,6,9-trimethyl-3-pentyl6a,7,8,10a-tetrahydro-6H-dibenzo[b,d]pyran-1-yl]oxy}butan-2-yl]amino}-4-oxobutanoic acid
4-{[(2S)-3-methyl-1-oxo-1-{[(6aR,10aR)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-dibenzo[b,d]pyran-1-yl]oxy}butan-2-yl]amino}-4-oxobutanoic acid
cannabinoid receptor agonist, DB 21741, XV9S3R9XJC
Suvadronabinol (DB21741) is a potent, synthetic small-molecule cannabinoid receptor type 1 (CB1) agonist, initially developed for therapeutic potential in areas like appetite stimulation, pain, or weight management, acting similarly to cannabis compounds but with specific design, currently in preclinical research stages, noted for its high selectivity and potency.
Key Characteristics:
- Type: Small Molecule Drug.
- Mechanism: A highly selective agonist for the cannabinoid receptor type 1 (CB1).
- Development: Originally developed by Elsohly Laboratories, it’s in preclinical R&D, with status as an experimental compound.
- Molecular Weight: Approximately 513.31 Da.
- CAS Number: 1225194-84-2.
Potential Applications (Research Areas):
- Appetite Stimulation & Weight Loss: Similar to dronabinol, it targets pathways involved in metabolism and appetite.
- Pain Management: As a cannabinoid, it interacts with the endocannabinoid system, which plays a role in pain perception.
Status:
- It’s an investigational compound, meaning it’s still under study and not yet approved for medical use.
In essence, Suvadronabinol is a targeted synthetic cannabinoid designed to interact with the body’s CB1 receptors, showing promise in preclinical research for conditions where cannabinoid effects are desired, but it’s not a widely available or established medicine.
SYN
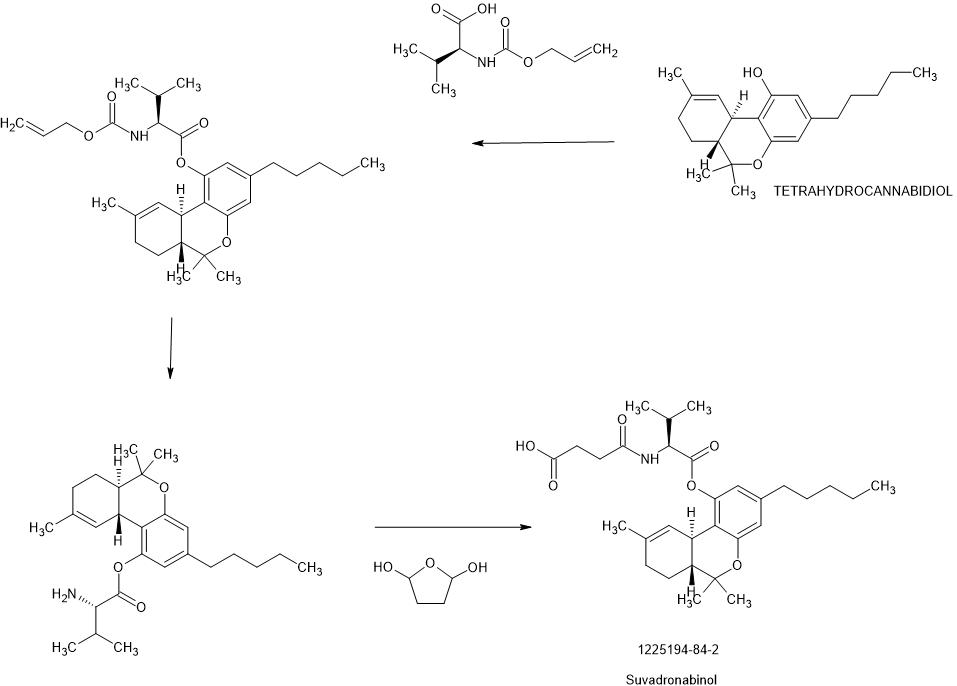
SYN
US20150045282
SYN
WO2010051541
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010051541&_cid=P22-MIXZ0J-96045-1
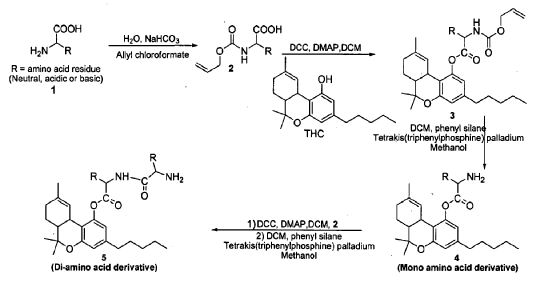
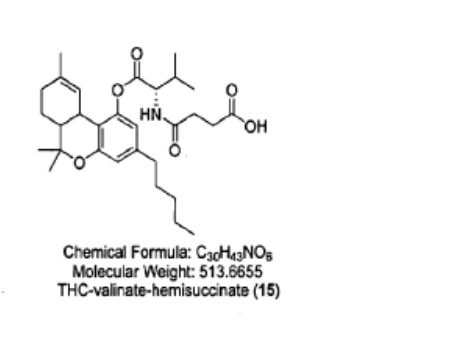
Example 10: Preparation of THC-valinate-hemisuccinate (15):
Compound 15 was also prepared using scheme II, where the starting material was compound 6 (THC-valinate). Product 15 was purified using column chromatography (>85% yield) and confirmed by mass spectroscopy in the positive ionization mode (M+NlV = 531) (Fig 17). The structure of product 15 was also confirmed by spectral analysis 1H-NMR and 13C-NMR (see Fig 18 for 13C-NMR assignments).
Spectral analysis of Δ9-THC prodrugs prepared above: Identity and purity of the synthesized prodrugs was established by spectral means including 1H-NMR, 13C-NMR and 2D-NMR such as COSY, HMQC, HMBC, as well as other spectroscopic means (IR1 UV and MS). The synthetic protocols outlined above yielded prodrugs with ≥95% purity.


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
////////Suvadronabinol, cannabinoid receptor agonist, DB 21741, XV9S3R9XJC














