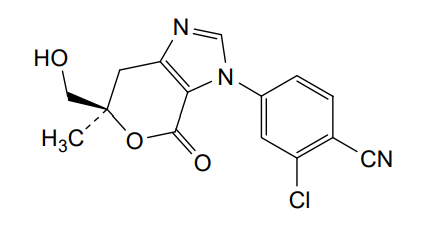
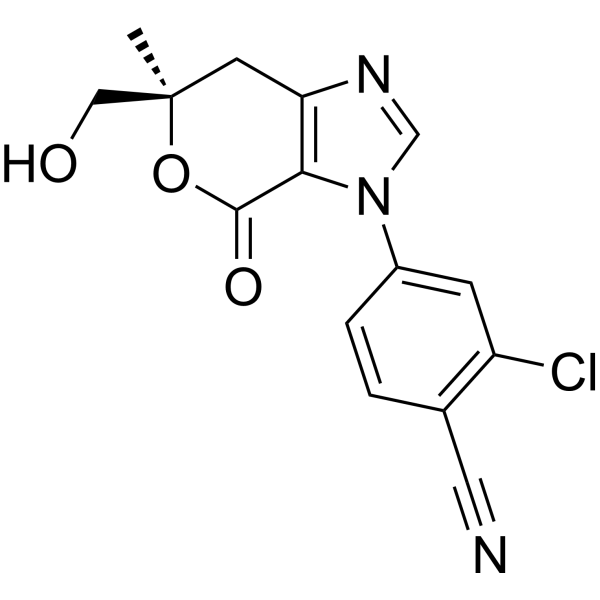
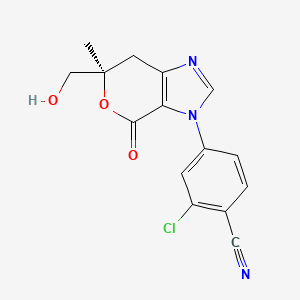
Vicadrostat
CAS 1868065-21-7
MF C15H12ClN3O3 MW 317.73
- 2-CHLORO-4-((6R)-6-(HYDROXYMETHYL)-6-METHYL-4-OXO-6,7-DIHYDROPYRANO(3,4-D)IMIDAZOL-3(4H)-YL)BENZONITRILE
- 2-Chloro-4-[(6R)-6,7-dihydro-6-(hydroxymethyl)-6-methyl-4-oxopyrano[3,4-d]imidazol-3(4H)-yl]benzonitrile
- 2-chloro-4-[(6R)-6-(hydroxymethyl)-6-methyl-4-oxo-7H-pyrano[3,4-d]imidazol-3-yl]benzonitrile
2-chloro-4-[(6R)-6-(hydroxymethyl)-6-methyl-4-oxo-6,7-dihydropyrano[3,4-d]imidazol-3(4H)-yl]benzonitrile
aldosterone synthase inhibitor, BI 690517, AF4VW4GA3H
Vicadrostat is an aldosterone synthase inhibitor (IC50=48 nM). Vicadrostat can be used for research in kidney diseases and cardiovascular diseases
Vicadrostat (BI 690517) is an investigational drug by Boehringer Ingelheim that selectively blocks aldosterone synthase, reducing excess aldosterone linked to kidney, heart, and metabolic diseases like chronic kidney disease (CKD) and heart failure. Currently in Phase III trials (EASi-KIDNEY and EASi-HF), it’s being tested alone and with empagliflozin (an SGLT2 inhibitor) to reduce proteinuria and improve heart/kidney health, showing promise in reducing albuminuria.
What it is
- Type: A highly selective Aldosterone Synthase Inhibitor (ASI).
- Mechanism: Blocks the enzyme that makes aldosterone, a hormone that causes fluid retention and damage in heart/kidney conditions.
What it’s for
- Conditions: Investigated for Chronic Kidney Disease (CKD) and Heart Failure with Preserved Ejection Fraction (HFpEF).
- Goal: To reduce high aldosterone levels, organ damage, and slow disease progression, particularly in interconnected cardiovascular and renal conditions.
How it’s being studied
- Combination Therapy: Key trials combine vicadrostat with empagliflozin (Jardiance).
- Promising Results: A Phase II trial showed significant reduction in urine protein (albuminuria) when combined with empagliflozin.
- Clinical Trials: Undergoing large Phase III trials (EASi-KIDNEY and EASi-HF) to confirm its efficacy and safety.
Key benefit
- Offers a potential new treatment by targeting aldosterone, addressing multiple interconnected organ systems (heart, kidney, metabolism) simultaneously.
- OriginatorBoehringer Ingelheim
- Class2 ring heterocyclic compounds; Alcohols; Benzonitrile; Chlorinated hydrocarbons; Imidazoles; Pyrones; Small molecules; Urologics
- Mechanism of ActionCytochrome P-450 CYP11B2 inhibitors
- Phase IIICardiovascular disorders; Heart failure; Hypertension; Renal failure; Type 2 diabetes mellitus
- No development reportedDiabetic nephropathies
- 28 Oct 2025No recent reports of development identified for phase-I development in Renal-failure(In volunteers) in Netherlands (IV)
- 28 Oct 2025No recent reports of development identified for phase-I development in Renal-failure(In volunteers) in Netherlands (PO)
- 08 Sep 2025Boehringer Ingelheim initiates a phase I trial (In volunteers, Combination therapy) in Germany (NCT07133399)
- A Study to Test Whether Vicadrostat in Combination With Empagliflozin Helps People With Chronic Kidney DiseaseCTID: NCT06926660Phase: Phase 2Status: RecruitingDate: 2025-11-28
- A Study to Test Whether Vicadrostat (BI 690517) in Combination With Empagliflozin Helps People With Heart Failure and a Weak Pumping Function of the Left Side of the HeartCTID: NCT06935370Phase: Phase 3Status: RecruitingDate: 2025-11-26
- A Study to Test Whether Vicadrostat in Combination With Empagliflozin Helps People With Heart FailureCTID: NCT06424288Phase: Phase 3Status: RecruitingDate: 2025-11-26
- A Study to Test Vicadrostat (BI 690517) Taken Together With Empagliflozin in People With Type 2 Diabetes, High Blood Pressure, and Cardiovascular DiseaseCTID: NCT07064473Phase: Phase 3Status: RecruitingDate: 2025-11-26
- A Study in Healthy Men to Compare the Amount of Vicadrostat and Empagliflozin in the Blood When Taken Separately and TogetherCTID: NCT07035457Phase: Phase 1Status: CompletedDate: 2025-08-20
SYN
compound 29 A [WO2016014736A1]
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016014736&_cid=P12-MJ3WOZ-69028-1
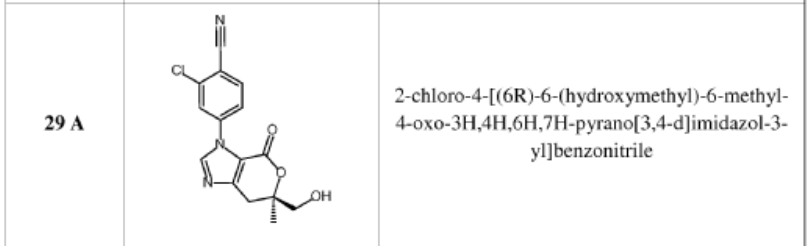
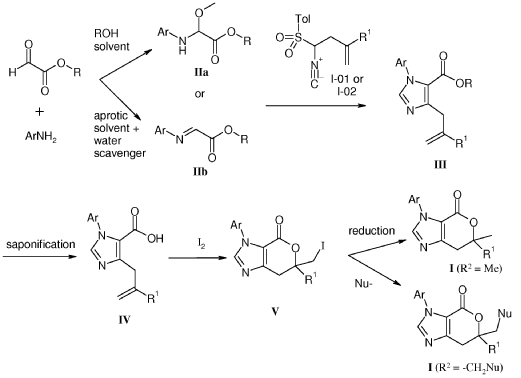
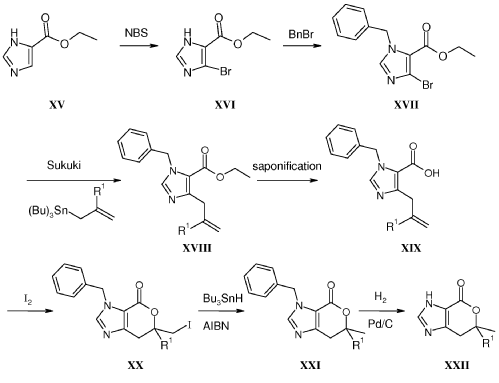
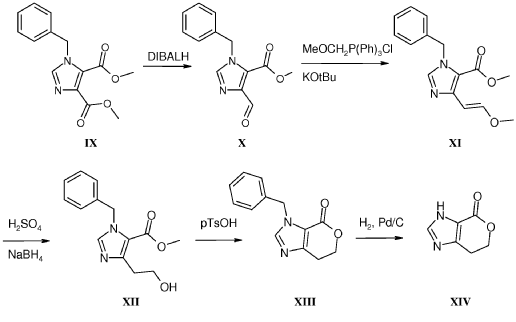
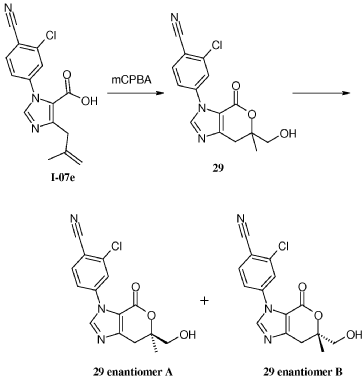
Example 8: Synthesis of 2-chloro-4-[(6R)-6-(hydroxymethyl)-6-methyl-4-oxo-3H,4H,6H,7H-pyrano[3,4-d]imidazol-3-yl]benzonitrile (29 enantiomer A) and 2-chloro-4-[(6S)-6-(hydroxymethyl)-6-methyl-4-oxo-3H,4H,6H,7H-pyrano[3,4-d]imidazol-3-yl]benzonitrile (29 enantiomer B)
29 enan
A mixture of 0.50 g (1.7 mmol) of I-07e and 0.56 g (2.5 mmol) of 77% m-CPBA (m-chloroperoxybenzoic acid) in 10 mL of CH2CI2 is stirred fori 6 h. EtOAc (200 mL) and 20 mL of 10% Na2S03 are added. The mixture is washed twice with 50 mL of NaHC03 and the washes are extracted with 50 mL of CH2C12. The organic extracts are combined, dried with MgS04, filtered and concentrated to give 507 mg of racemic 29 as a pale yellow solid. Chiral
chromatography of 507 mg (LUX 5u Cellulose 4, 28% EtOH:C02, 80 g/min, 120 bar, 40 °C) delivers 238 mg of 29 enantiomer A and 230 mg of 29 enantiomer B. The absolute
stereochemistry for compounds 29 A and 29 B were determined by high resolution single crystal X-ray crystallography structure determination and careful examination of the Flack parameter on the refined structures (H.D. Flack and G. Bernardinelli, 2008, Chirality, 20, 681-690).
The following compounds are prepared from the appropriate olefin I-07c and n in the same manner as 29 enantiomers A & B.
3- (3,4-dichlorophenyl)-6-(hydroxymethyl)-6-methyl-3H,4H,6H,7H-pyrano[3,4- d]imidazol-4-one (30 enantiomers A & B) from I-07c.(RegisPack, 25% (EtOH + 1% iPrNH2):C02, 80 mL/min, 100 bar, 25 °C)
4- [6-(hydroxymethyl)-6-methyl-4-oxo-3H,4H,6H,7H-pyrano[3,4-d]imidazol-3-yl]-3- methylbenzonitrile (31 enantiomers A & B) from I-07n. (LUX 5u Cellulose 4, 25% EtOH:C02, 90 g/min, 120 bar, 40 °C)
SYN
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2025174790&_cid=P12-MJ3WOZ-69028-1
PAT
- Aldosterone synthase inhibitorsPublication Number: US-2016024105-A1Priority Date: 2014-07-24
- Aldosterone synthase inhibitorsPublication Number: US-9334285-B2Priority Date: 2014-07-24Grant Date: 2016-05-10
- Aldosterone synthase inhibitorsPublication Number: WO-2016014736-A1Priority Date: 2014-07-24
- Aldosterone synthase inhibitorsPublication Number: KR-102378648-B1Priority Date: 2014-07-24Grant Date: 2022-03-28
- Aldosterone synthase inhibitors for treating chronic kidney diseasePublication Number: US-2025049763-A1
- Aldosterone synthase inhibitors for treating chronic kidney diseasePublication Number: US-2023181538-A1Priority Date: 2021-12-14
- Aldosterone synthase inhibitorsPublication Number: EP-3172212-A1Priority Date: 2014-07-24
- Aldosterone synthase inhibitorsPublication Number: EP-3172212-B1Priority Date: 2014-07-24Grant Date: 2018-06-13
- Aldosterone synthase inhibitorPublication Number: JP-2017521466-APriority Date: 2014-07-24
- Aldosterone synthase inhibitorPublication Number: JP-6250862-B2Priority Date: 2014-07-24Grant Date: 2017-12-20


AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
[1]. Jennifer Burke, et al. Aldosterone synthase inhibitors.WO2016014736.2018-09-07
- Targeting aldosterone to improve cardiorenal outcomes: from nonsteroidal mineralocorticoid receptor antagonists to aldosterone synthase inhibitorsPublication Name: Current opinion in nephrology and hypertensionPublication Date: 2025-02-27PMID: 40012539DOI: 10.1097/mnh.0000000000001067
- The potential for improving cardio-renal outcomes in chronic kidney disease with the aldosterone synthase inhibitor vicadrostat (BI 690517): a rationale for the EASi-KIDNEY trialPublication Name: Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal AssociationPublication Date: 2024-11-12PMCID: PMC12209857PMID: 39533115DOI: 10.1093/ndt/gfae263
- Emerging Therapies for Treatment-Resistant Hypertension: A Review of Lorundrostat and Related Selective Aldosterone Synthase InhibitorsPublication Name: Cardiology in ReviewPublication Date: 2024-02-15PMID: 38358268DOI: 10.1097/crd.0000000000000665
- Author response for ‘Aldosterone synthase inhibitor (BI 690517) therapy for people with diabetes and albuminuric chronic kidney disease: A multicentre, randomized, double‐blind, placebo‐controlled, Phase I trial’Publication Name: Diabetes, Obesity and MetabolismPublication Date: 2024-01-16PMID: 38497241DOI: 10.1111/dom.15518
//////////vicadrostat, aldosterone synthase inhibitor, BI 690517, AF4VW4GA3H














