
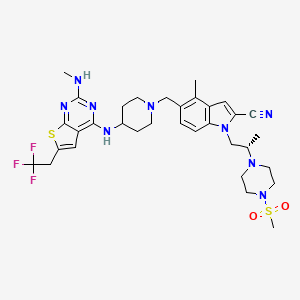
Ziftomenib
CAS 2134675-36-6
4MOD1F4ENC, KO 539
717.9 g/mol, C33H42F3N9O2S2
APPROVALS 2025, FDA 2025, 11/13/2025, Komzifti
4-methyl-5-[[4-[[2-(methylamino)-6-(2,2,2-trifluoroethyl)thieno[2,3-d]pyrimidin-4-yl]amino]piperidin-1-yl]methyl]-1-[(2S)-2-(4-methylsulfonylpiperazin-1-yl)propyl]indole-2-carbonitrile
To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible nucleophosmin 1 mutation who have no satisfactory alternative treatment options
Ziftomenib, sold under the brand name Komzifti, is an anti-cancer medication used for the treatment of acute myeloid leukemia.[1] Ziftomenib is a menin inhibitor.[1] It is taken by mouth.[1]
Ziftomenib blocks the interaction between two proteins, menin (MEN1) and KMT2A (also known as mixed lineage leukemia protein, MLL).[2][3]
Ziftomenib was approved for medical use in the United States in November 2025.[4][5]
Ziftomenib, also known as KO539, is an orally bioavailable inhibitor of the menin-mixed lineage leukemia (MLL; myeloid/lymphoid leukemia; KMT2A) fusion protein, with potential antineoplastic activity. Upon oral administration, ziftomenib prevents the interaction between the two proteins menin and MLL, and thus the formation of the menin-MLL complex. This reduces the expression of downstream target genes and results in an inhibition of the proliferation of MLL-rearranged leukemic cells. The menin-MLL complex plays a key role in the survival, growth and proliferation of certain kinds of leukemia cells
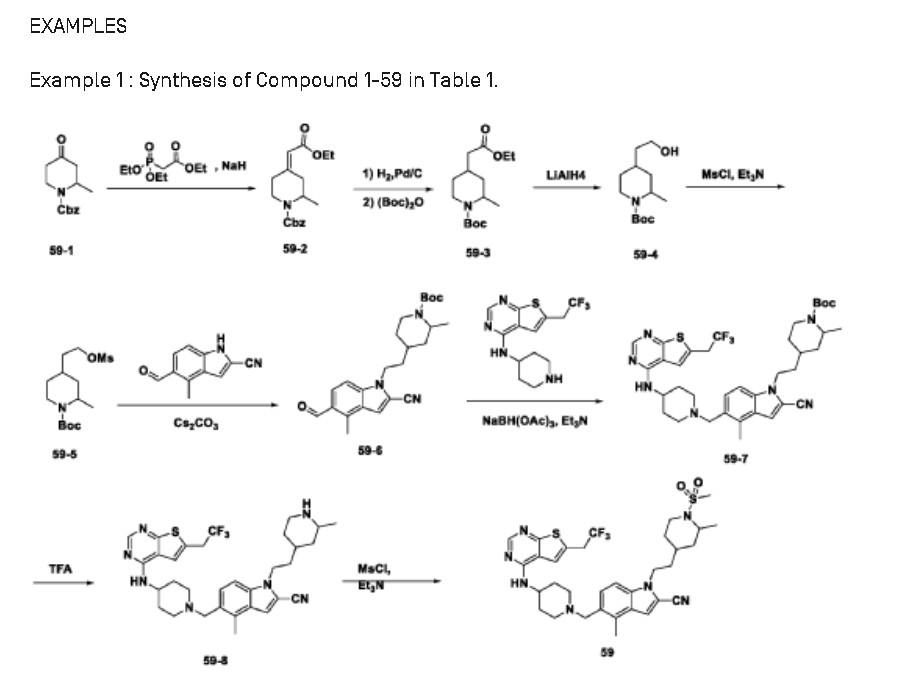
SYN

syn
WO2022086986
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022086986&_cid=P11-MI9OM2-05631-1

above similar not same
pat
WO2020069027
WO2018175746
WO2017161028
WO2018106820
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US239825810&_cid=P20-MI88RV-91969-1

PAT
- Inhibitors of the myst family of lysine acetyl transferasesPublication Number: US-2025051343-A1
- Small molecule inhibitors of dyrk/clk and uses thereofPublication Number: US-2025051325-A1
- VCP/p97 INHIBITOR FOR THE TREATMENT OF CANCERPublication Number: US-2025049800-A1
- RNAi agents for inhibiting expression of HIF-2 alpha (EPAS1), compositions thereof, and methods of usePublication Number: US-12221610-B2Grant Date: 2025-02-11
- Inhibitors of the myst family of lysine acetyl transferasesPublication Number: US-2025051343-A1
- Small molecule inhibitors of dyrk/clk and uses thereofPublication Number: US-2025051325-A1
- VCP/p97 INHIBITOR FOR THE TREATMENT OF CANCERPublication Number: US-2025049800-A1
- RNAi agents for inhibiting expression of HIF-2 alpha (EPAS1), compositions thereof, and methods of usePublication Number: US-12221610-B2Grant Date: 2025-02-11
- N-(3-hydroxy-4-piperidinyl)benzamide derivatives and pharmaceutical compositionsPublication Number: NZ-201856-APriority Date: 1981-10-01
- Novel n-(3-hydroxy-4-piperidinyl)benzamide derivativesPublication Number: EP-0076530-B1Priority Date: 1981-10-01Grant Date: 1985-12-11
- Novel N-(3-hydroxy-4-piperidinyl)benzamide derivativesPublication Number: EP-0076530-A2Priority Date: 1981-10-01
- Boron-containing polyphosphonates for the treatment of calcogenic tumorsPublication Number: JP-S5817120-APriority Date: 1981-06-30
- Novel n-aryl-piperazinealkanamidesPublication Number: IE-53465-B1Priority Date: 1981-06-23
- Novel((bis(aryl)methylene)-1-piperidinyl)-alkyl-pyrimidinonesPublication Number: IE-56180-B1Priority Date: 1982-11-01
- Novel ((bis(aryl)methylene)-1-piperidinyl)alkyl-pyrimidinonesPublication Number: EP-0110435-B1Priority Date: 1982-11-01Grant Date: 1989-01-04
- Novel ((bis(aryl)methylene)-1-piperidinyl)alkyl-pyrimidinonesPublication Number: EP-0110435-A1Priority Date: 1982-11-01
- NEW // BIS (ARYL) METHYLENE / -1-PIPERIDINYL / -ALKYL-PyrimidinonesPublication Number: BG-60538-B2Priority Date: 1982-11-01
- Process for preparing n-(3-hydroxy-4-piperidinyl)benzamide derivativesPublication Number: KR-860001584-B1Priority Date: 1982-07-30Grant Date: 1986-10-10



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
Medical uses
Ziftomenib is indicated for the treatment of adults with relapsed or refractory acute myeloid leukemia with a susceptible nucleophosmin 1 mutation who have no satisfactory alternative treatment options.[1]
Adverse effects
The US prescribing information includes warnings and precautions for differentiation syndrome, QTc interval prolongation, and embryo-fetal toxicity.[4]
History
Efficacy was evaluated in KO-MEN-001 (NCT04067336), an open-label, single, arm, multi-center trial in 112 adults with relapsed or refractory acute myeloid leukemia with an nucleophosmin 1 mutation identified using next-generation sequencing or polymerase chain reaction.[4] Participants with nucleophosmin 1 mutations, including type A, B, and D mutations and other nucleophosmin 1 mutations likely to result in cytoplasmic localization of the nucleophosmin 1 protein, were enrolled.[4]
The US Food and Drug Administration granted the application for ziftomenib priority review, breakthrough therapy, and orphan drug designations.[4]
Society and culture
Legal status
Ziftomenib was approved for medical use in the United States in November 2025.[6]
Names
Ziftomenib is the international nonproprietary name.[7][8]
Ziftomenib is sold under the brand name Komzifti.[6]
References
- https://kuraoncology.com/wp-content/uploads/prescribinginformation.pdf
- “Ziftomenib”. NCI Cancer Dictionary. National Cancer Institute.
- Rausch J, Dzama MM, Dolgikh N, Stiller HL, Bohl SR, Lahrmann C, et al. (October 2023). “Menin inhibitor ziftomenib (KO-539) synergizes with drugs targeting chromatin regulation or apoptosis and sensitizes acute myeloid leukemia with MLL rearrangement or NPM1 mutation to venetoclax”. Haematologica. 108 (10): 2837–2843. doi:10.3324/haematol.2022.282160. PMC 10543165. PMID 37102614.
- “FDA approves ziftomenib for relapsed or refractory acute myeloid leukemia with a NPM1 mutation”. U.S. Food and Drug Administration (FDA). 13 November 2025. Retrieved 14 November 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - “Novel Drug Approvals for 2025”. U.S. Food and Drug Administration (FDA). 13 November 2025. Retrieved 14 November 2025.
- “Kura Oncology and Kyowa Kirin Announce FDA Approval of Komzifti (ziftomenib), the First and Only Once-Daily Targeted Therapy for Adults with Relapsed or Refractory NPM1-Mutated Acute Myeloid Leukemia” (Press release). Kura Oncology. 13 November 2025. Retrieved 14 November 2025 – via GlobeNewswire News Room.
- World Health Organization (2022). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 87”. WHO Drug Information. 36 (1). hdl:10665/352794.
- World Health Organization (2022). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 88”. WHO Drug Information. 36 (3). hdl:10665/363551.
Further reading
- Wang ES, Issa GC, Erba HP, Altman JK, Montesinos P, DeBotton S, et al. (October 2024). “Ziftomenib in relapsed or refractory acute myeloid leukaemia (KOMET-001): a multicentre, open-label, multi-cohort, phase 1 trial”. The Lancet. Oncology. 25 (10): 1310–1324. doi:10.1016/S1470-2045(24)00386-3. PMID 39362248.
External links
- Clinical trial number NCT04067336 for “First in Human Study of Ziftomenib in Relapsed or Refractory Acute Myeloid Leukemia” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Trade names | Komzifti |
| Other names | KO-539; KO539 |
| AHFS/Drugs.com | Komzifti |
| License data | US DailyMed: Ziftomenib |
| Routes of administration | By mouth |
| Drug class | Antineoplastic |
| ATC code | None |
| Legal status | |
| Legal status | US: ℞-only[1] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2134675-36-6 |
| PubChem CID | 138497449 |
| IUPHAR/BPS | 11680 |
| DrugBank | DB17171 |
| ChemSpider | 115009296 |
| UNII | 4MOD1F4ENC |
| KEGG | D12419 |
| ChEMBL | ChEMBL5095038 |
| PDB ligand | K5O (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C33H42F3N9O2S2 |
| Molar mass | 717.88 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
////////Ziftomenib, APPROVALS 2025, FDA 2025, 4MOD1F4ENC, Komzifti














