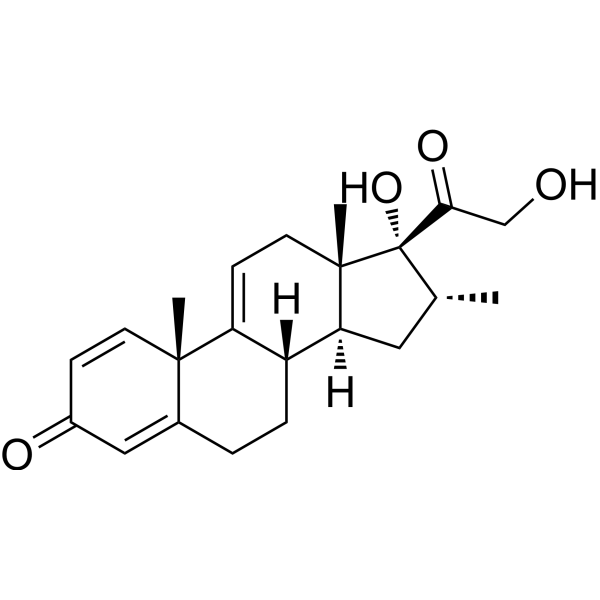
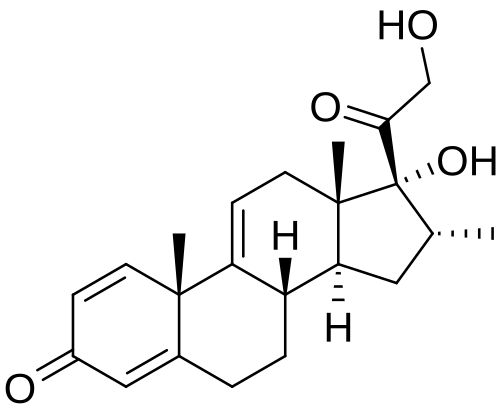
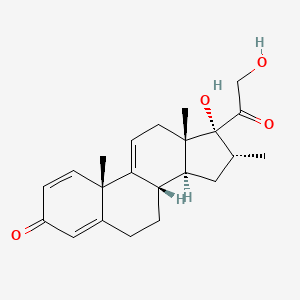

Vamorolone
CAS 13209-41-1
| Molecular Weight | 356.46 |
|---|---|
| Formula | C22H28O4 |
- Agamree
- 17,21-Dihydroxy-16alpha-methylpregna-1,4,9(11)-triene-3,20-dione
- VBP-15 free alcohol
- (8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one
- (16alpha)-17,21-dihydroxy-16-methylpregna-1,4,9(11)-triene-3,20-dione
- (8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta(a)phenanthren-3-one
- DTXCID601356317
- (1R,2R,3aS,3bS,9aS,11aS)-1-hydroxy-1-(2-hydroxyacetyl)-2,9a,11a-trimethyl-1H,2H,3H,3aH,3bH,4H,5H,7H,9aH,11H,11aH-cyclopenta(a)phenanthren-7-one
Vamorolone (VBP15) is a first-in-class, orally active dissociative steroidal anti-inflammatory agent and membrane-stabilizer. Vamorolone improves muscular dystrophy without side effects. Vamorolone shows potent NF-κB inhibition and substantially reduces hormonal effects.
Vamorolone, sold under the brand name Agamree, is a synthetic corticosteroid, which is used for the treatment of Duchenne muscular dystrophy.[4][5][6][7][8] It is taken by mouth.[1] It is a dual atypical glucocorticoid and antimineralocorticoid.[9]
The most common adverse reactions include cushingoid features, psychiatric disorders, vomiting, increased weight, and vitamin D deficiency.[10]
Vamorolone was approved for medical use in the United States in October 2023,[11][10] and in the European Union in December 2023.[2][3]
Vamorolone is a novel and fully synthetic glucocorticoid developed by Santhera Pharmaceuticals. It is used to manage inflammation and immune dysregulation in patients with Duchenne muscular dystrophy (DMD), a neuromuscular disorder characterized by the insidious regression of neuromuscular function and the most common form of muscular dystrophy in the United States. Corticosteroid therapy is the current standard of care for DMD despite relatively high rates of adverse effects. Vamorolone is positioned as having a more tolerable adverse effect profile than other corticosteroids owing to its unique receptor binding profile, thus providing an additional treatment option in patients for whom corticosteroid adverse effects are intolerable or otherwise unacceptable. Vamorolone was approved by the FDA in October 2023 for the management of DMD in patients ≥2 years of age. In December 2023, it was approved in the EU for the treatment of patients ≥4 years of age.
PATENT
https://patents.google.com/patent/WO2023016817A1/en
Vamorolone is currently produced from the commercially available 3TR (Tetraene acetate) – see Scheme 2. In step a, TMS imidazole, MeMgCI and THF are added to 3TR, with subsequent addition of CuAc2, H2O, DMPU, MeMgCI and THF in step b. Under treatment with peracetic acid in Toluene from compound 2 the intermediate 3 is formed in step c. After treatment with NaHSCO3 and TFA (step d), EtOAc and heptane (step e) and acetonitrile trituration (step f) HBr in CH2CI2 is added (step g) and MeOH (step h) is used for crystallization to form Acetyl- Vamorolone 4. Acetyl-Vamorolone is deacetylated with K2CO3 in MeOH, followed by HCI to obtain Vamorolone (step i). The synthesis is disclosed in Bioorganic & Medicinal Chemistry, Volume 21 , Issue 8, 15 April 2013, Pages 2241-2249.
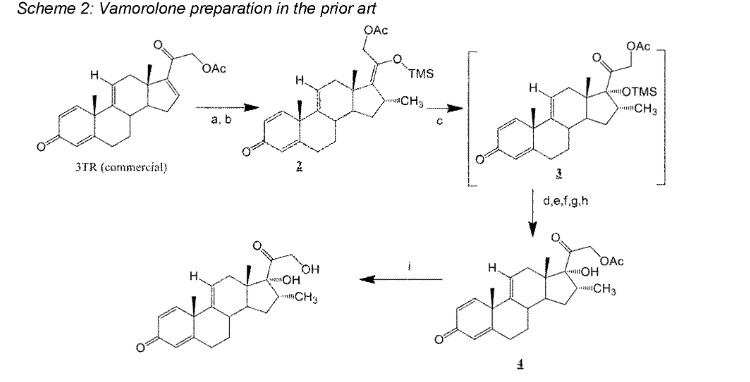
Example 2: Synthesis of the present invention
Scheme C: Route of Synthesis of Vamorolone from 8-DM

Vamorolone was synthesized in three synthetic steps from commercially available 8-DM.
The synthetic route started with the acetylation of 8-DM using acetic anhydride and catalytic DMAP in THF, followed by crystallization of 8-DM Acetate after aqueous quench. Then, a deoxygenation reaction converted 8-DM Acetate directly into Vamorolone Acetate. This deoxygenation proceeded via initial formation of an iodohydrin with excess aq. HI, followed by simultaneous I2 and H2O-elimination to give Vamorolone Acetate. During the reaction, partial de-acetylation occurred (20-25%) and therefore re-acetylation with acetic anhydride was necessary. After completed re-acetylation, Vamorolone Acetate was directly crystallized by addition of H2O. Finally, the acetate group is cleaved under basic conditions to give crude Vamorolone, which was recrystallized from iPrOH to obtain the pure product.
2.1 Acetylation

A 10 L glass dj ( double jacketed reactor )-reactor was charged with 8-DM (490 g, 1.32 mol, 1.0 eq.) and DMAP (16.1 g, 0.132 mmol, 0.10 eq.). THF (1.25 L, 2.5 vol.) was added at IT = 20-25 °C. Then, AC2O (201 g, 187 mL, 1.97 mol, 1.5 eq.) was added dropwise over 20-40 min, keeping IT below 30 °C during the addition. After complete addition, the reaction mixture was stirred at IT = 20-25 °C for 30 min. IPC control by LC/MS indicated >99% conversion of 8-DM to 8-DM Acetate.
The reaction mixture was quenched by dropwise addition of H2O (4.9 L, 10 vol.) over 30-45 min, keeping IT below 25 °C. The resulting aqueous suspension was aged at IT = 20-25 °C for 1 h. The product was filtered off, washed with H2O (3 x 0.5 L), and dried on a rotary evaporator (900-10 mbar, 65 °C bath temperature) to provide 8-DM Acetate (539 g, 1.30 mol, 99% yield, >99% a/a, 98% w/w) as a white solid (cryst 1#1).
Analytical Data:
LC/MS column: Zorbax RRHD SB-Aq, 2.1x50mm, 1.8pm
Program: G_005%B_TFA_0,800ml_2,00min
Eluent A: Water/TFA 100:0.04, Eluent B: Acetonitrile
IPC preparation for LC/MS
10 microliter in 1 mL H2O:MeCN 1 :1
Conversion was determined with respect to consumption of 8-DM relative to formation of 8- DM Acetate.
Detected mass: [M+1]= 373.19 for 8-DM and [M+1] = 415,19 8-DM Acetate. 2.2 Deoxygenation with HI

A 10 L glass dj-reactor was charged with 8-DM Acetate (500 g, 1.21 mol, 1.0 eq.). Toluene (2.5 L, 5 vol.) was added. The suspension was cooled to IT = 0-5 °C and then a solution of 57% aqueous HI (1 .08 kg, 637 mL, 4.83 mol, 4.0 eq.) in AcOH (1.25 L, 2.5 vol.) was added via peristaltic pump over 45-60 min, keeping IT below 5 °C during the addition. The resulting dark purple to brown solution was stirred at IT = 3-5 °C for 24 h. IPC control by LC/MS indicated >98% conversion of 8-DM Acetate/intermediate iodohydrin to Vamorolone Acetate/Vamorolone.
The reaction mixture was quenched by dropwise addition of 25% aq. Na2SO3 solution (2.0 L, 4 vol.) over 10-20 min, keeping IT below 15 °C. After complete addition, EtOAc (1.0 L, 2 vol.) was added and the biphasic mixture was warmed to IT = 15-20 °C. Stirring was stopped and the phases were separated (Organic Phase 1 and aqueous Phase 1 ; goal pH of the aqueous Phase 1 : 2; aqueous Phase 1 disposed). 25% aq. Na2SO3 solution (1.25 L, 2.5 vol.) was added to Organic Phase 1 and the biphasic mixture was stirred at IT = 15-20 °C for 5 min, stirring was stopped and phases separated (Organic Phase 1 and aqueous Phase 2; goal pH aqueous Phase 2: 4-5; aqueous Phase 2 disposed). 25% aq. Na2SO3 solution (1.25 L, 2.5 vol.) was added to Organic Phase 1 and the biphasic mixture was stirred at IT = 15-20 °C for 5 min, stirring was stopped and phases separated (Organic Phase 1 and aqueous Phase 3; goal pH aqueous Phase 3: 5-6; aqueous Phase 3 disposed). H2O (0.5 L, 1.0 vol.) was added to Organic Phase 1 and the biphasic mixture was stirred at IT = 15-20 °C for 5 min, stirring was stopped and phases separated (Organic Phase 1 and aqueous Phase 4; goal pH aqueous Phase 4: 5-6; aqueous Phase 4 disposed).
A slight vacuum was applied to the double-jacketed reactor (100-150 mbar), containing Organic Phase 1 , and toluene was distilled off at 70 °C jacket temperature (ET) from the reaction mixture with continuous addition of MeCN, and the distillation continued until target residual toluene value has been reached (goal: less than 5% toluene according to 1 H-NMR of reaction mixture. Final volume in reactor after distillation: ca. 3.5 L (7.5 vol.).
Once toluene was removed, the vacuum was broken with N2 and resulting fine suspension cooled to IT = 20-25 °C. At this point, the amount of Vamorolone was assessed by IPC (typical ratio: Vamorolone Acetate to Vamorolone: 75:25; x = 25% a/a). DMAP (3.7 g, 0.0302 mol, 0.025 eq.) was added, followed by slow addition of AC2O (61.6 g, 57 mL, 0.603 mol, 0.5 eq.) over 5-10 min at IT = 20-25 °C. After complete addition of AC2O, the reaction mixture was stirred for 30 min at IT = 20-25 °C. IPC control by LC/MS indicated ≤ 2% a/a Vamorolone (ratio: Vamorolone Acetate to Vamorolone: 98.5:1.5).
The reaction mixture was quenched by slow addition of H2O (4.9 L, 10 vol.) over 15-30 min, keeping IT below 25 °C. The resulting aqueous suspension was cooled to IT = 0-5 °C and aged at this temperature for 2 h. The product was filtered off, washed with H2O/MeCN 4:1 (2 x 0.5 L), and dried on a rotary evaporator (900-10 mbar, 65 °C bath temperature) to provide Vamorolone Acetate (301 g, 0.76 mol, 63% yield, 98% a/a, 98% w/w) as an off-white solid (cryst 1#1).
Over the course of the reaction, partial de-acetylation of Vamorolone Acetate to Vamorolone was observed (between 20-25% a/a). Therefore, after aq. workup and solvent switch to MeCN, the ratio of Vamorolone Acetate to Vamorolone was assessed by LC/MS (in % a/a), and the following amounts of DMAP and Ac2O were added: x = amount of Vamorolone in % a/a (e.g. x = 20% a/a)
DMAP eq. = (0.1 -x)/100 (e.g. 0.02 eq.)
Ac2O eq. = (2.0-x)/100 (e.g. 0.40 eq.)
Analytical Data
LC/MS column: Zorbax RRHD SB-Aq, 2.1x50mm, 1.8pm
Program: G_005%B_TFA_0,800ml_2,00min
Eluent A: Water/TFA 100:0.04, Eluent B: Acetonitrile
IPC preparation for LC/MS
10 microliter in 1 mL H2O:MeCN 1 :1
Conversion was determined with respect to consumption of the sum of (8-DM Acetate + intermediate iodohydrin) relative to the sum of (Vamorolone Acetate + Vamorolone). Detected mass: [M+1] = 415,19 for 8-DM Acetate, [M+1]= 357,28 for Vamorolone, 399,20 for
Vamorolone Acetate and 543,12 for intermediate lodohydrin
2.3 De-Acetylation

A 10 L glass dj-reactor was charged with Vamorolone Acetate (280 g, 0.703 mol, 1.0 eq.). MeOH (1.54 L, 5.5 vol.) was added. The suspension was cooled to IT = 0-5 °C and then a solution of K2CO3 (107 g, 0.773 mol, 1.1 eq.) in H2O (0.7 L, 2.5 vol.) was added dropwise via peristaltic pump over 20-40 min, keeping IT below 10 °C during the addition. After complete addition, the reaction mixture was warmed IT = 20-25 °C and stirred for 5 h. IPC control by LC/MS indicated 99.3% conversion of Vamorolone Acetate to Vamorolone.
The reaction mixture was cooled to IT = 15-17 °C and quenched by dropwise addition of 1 M aq. HCI (950 mL, 0.95 mol, 1.35 eq.) over 20-40 min, keeping IT below 20 °C during the addition (goal pH: 5-6). The resulting aqueous suspension was aged at IT = 15-20 °C for 12 h. The product was filtered off, washed with H2O/MeOH 2:1 (3 x 0.3 L), and dried on a rotary evaporator (900-10 mbar, 65 °C bath temperature) to provide Vamorolone (241.5 g, 0.68 mol, 96% yield, >99% a/a, 98% w/w) as a slightly yellow solid (crude 1#1).
Analytical Data
LC/MS column: Zorbax RRHD SB-Aq, 2.1x50mm, 1.8pm
Program: G_005%B_TFA_0,800ml_2,00min
Eluent A: Water/TFA 100:0.04, Eluent B: Acetonitrile
IPC preparation for LC/MS
10 microliter in 1 mL H2O:MeCN 1 :1
Conversion was determined with respect to consumption of Vamorolone Acetate relative to formation of Vamorolone. 2.4 Recrystallization

A 10 L glass dj-reactor was charged with Vamorolone (230 g, 0.645 mol, 1.0 eq.). iPrOH (5 L, 22 vol.) was added. The suspension was heated to reflux (jacket temperature ET = 97 °C) and stirred until complete dissolution of Vamorolone occurred (10-15 min on this scale).
After complete dissolution, the clear yellow solution was slowly cooled to IT = 0-5 °C over the course of 12 h and then aged at IT = 0-5 °C for 1 h. The recrystallized product was filtered off, washed with cold iPrOH (2 x 250 mL), and dried on a rotary evaporator (900-10 mbar, 65 °C bath temperature) to provide Vamorolone (201 g, 87% recovery, >99% a/a, 99% w/w) as an off white glimmery solid (cryst 1#1).
Iso-propanol (iPrOH) was found to the best solvent for recrystallization with excellent purity upgrading properties (by rejection of impurities), although a high dilution is necessary to completely dissolve the crude Vamorolone at reflux temperature. Higher concentrations for the recrystallization satisfactory results are obtainable using mixtures of isopropanol and water. Maximum solubility of Vamorolone was determined to be at reflux of a 80:20 (isopropanol : water) mixture.
PATENT
https://patents.google.com/patent/US20200281942A1/en
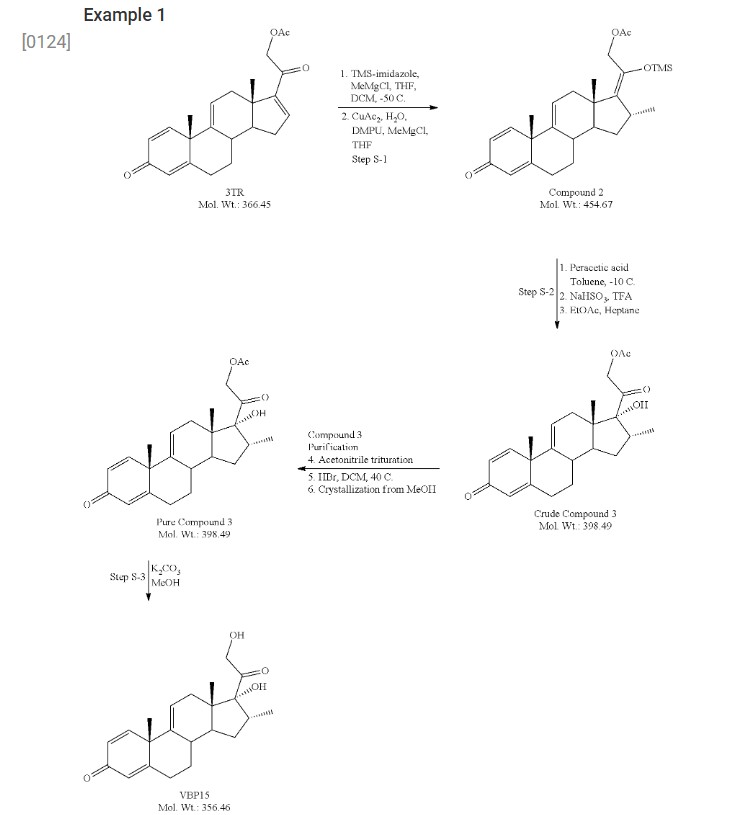
- [0124]
- [0125]3-TR (100 g, 273 mmol), dichloromethane (DCM, 500 mL) and tetrahydrofuran (THF, 400 mL) were charged to a reaction flask under nitrogen. To this was charged trimethylsilyl imidazole (TMS-imidazole, 65.3 g, 466 mmol, 1.7 eq). The resulting mixture was stirred at room temperature for 3 hours.
- [0126]In a separate flask, copper acetate monohydrate (5.4 g, 27 mmol), tetrahydrofuran (400 ml) and 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU, 53.3 g, 416 mmol) were combined and stirred at room temperature for approximately 3 hours. The blue mixture was subsequently cooled to −50° C., and to this was added methyl magnesium chloride solution (27 ml, 3.0 M in THF, 82 mmol) dropwise. After 30 minutes, the mixture had formed a deep blue, sticky “ball.”
- [0127]The 3-TR/TMS-imidazole mixture was cooled to −50° C. and to this was charged the copper acetate/DMPU solution above via canula. The residual sticky mass from the copper acetate/DMPU mixture was dissolved using DCM (50 mL) and also transferred.
- [0128]Methyl magnesium chloride (123.2 mL, 3.0 M solution in THF, 368 mmol) was added dropwise over 45 minutes to the combined reaction mixtures, which were then allowed to stir for 2 hours at −50° C. Subsequent HPLC analysis showed complete consumption of starting material. The mixture was allowed to warm to room temperature overnight, with stirring.
- [0129]Toluene (800 mL) was added to the mixture, followed by 5% acetic acid solution (600 mL). The aqueous layer was removed and discarded. The acetic acid wash was repeated. The organic layer was washed with brine (400 mL), 5% sodium bicarbonate solution (400 mL×2), followed by a brine wash (400 mL). The organic solution was dried over sodium sulfate, then concentrated to dryness under reduced pressure. The product was recovered as a viscous, light golden oil. Mass recovery was 146 grams (119% of theoretical).
- [0130]Compound 2 (92 g, 202 mmol) and toluene (1000 mL, 10.9 vol) were charged to a reaction flask under nitrogen and the solution was cooled to −10° C. A 32 wt % solution of peracetic acid in acetic acid (60 mL, 283 mmol, 1.4 eq) was added dropwise over about 30 min maintaining the temperature at −10° C. The reaction was held for approximately 20 h (HPLC showed 75% Cmpd 3, Cmpd 2 1.5%, 6% diastereomer; 5% epoxide). Starting at −10° C., a 20% aqueous solution of sodium bisulfite (920 mL, 10 vol) was added carefully via addition funnel, keeping the temperature below 10° C. Trifluoroacetic acid (16 mL, 202 mmol, 1 eq) was added and the mixture was held for 3 h at 0-5° C. to complete desilylation (endpoint by HPLC). The lower aqueous layer was drained, and the organic layer was washed with a saturated solution of sodium bicarbonate (3×250 mL), followed by water (1×250 mL), and brine (1×150 mL). The organic layer was then dried over Na2SO4, filtered and concentrated to a pasty solid (89 g). The residue was taken up in 1.5 vol of EtOAc and transferred to neat heptane (19 vol) to precipitate crude Cmpd 3 as an off-white solid (50 g, 62.5% yield; HPLC 79% Cmpd 3, 5.6% epoxide, 1.7% diastereomer). The crude Cmpd 3 (48.5 g) was triturated in hot acetonitrile (2 vol) at 60° C. for 4 h, and then gradually cooled to ambient temperature overnight. The mixture was filtered using the recycled filtrate to rinse and wash the wet cake. After drying, the recovery was 64.3% (31.2 g; HPLC 93.5% Cmpd 3, 3.3% epoxide). To remove the epoxide impurity, the 31 Cmpd 3 was dissolved in DCM (250 mL, 8 vol) and a solution of 48% HBr in water was added (7.5 mL). The mixture was heated at 40° C. for 1 h (HPLC<0.3% epoxide). The mixture was cooled and transferred to a separatory funnel. The lower aqueous layer (brown) was removed and the upper organic layer was washed with water (200 mL), saturated NaHCO3 (150 mL), and brine (100 mL). The organic layer was dried over Na2SO4, filtered, and concentrated to a tan foam (32 g, ˜100% recovery). Methanol (64 mL, 2 vol) was added to the 32 g foam forming a slurry. To this was added a 1:1 solution of MeOH:water (60 mL, 2 vol) dropwise. The slurry cooled to slightly below ambient temperature and filtered using recycled filtrate to rinse and wash the wet cake. The solids were dried to constant weight, affording 26.1 g Cmpd 3 (81% recovery; HPLC 97.8%). The overall yield for Step 2 was 32.5%.
- [0131]Compound 3 (26 g, 65 mmol) and MeOH (156 mL, 6 vol) were mixed in a reaction flask and cooled to 0-5° C. A solution of K2CO3 (9.9 g, 72 mmol, 1.1 eq) in water (65 mL) was added dropwise, and the mixture was allowed to gradually warm to ambient temperature overnight. Analysis by HPLC showed 2.5% SM and another 5 mol % K2CO3 was added and the mixture stirred for another day (HPLC endpoint 1.1% Cmpd 3). The mixture was neutralized to pH 7 with 1.5 M HCl (53 mL) and ˜25% of the MeOH (30 g) was removed under vacuum to maximize recovery. After stirring for 2 days, the product was isolated by filtration using the recycled filtrate to aid transferring the wet cake to the funnel. The wet cake was dried under vacuum, affording 19.3 g VBP15 (83% yield) as an off-white powder. Analysis of the solids by HPLC showed 98.8% purity with 0.6% Cmpd 3 as the only major impurity.
- [0132]Power X-Ray Diffraction (pXRD)
- [0133]The solid samples were examined using X-ray diffractometer (Bruker D8 advance). The system is equipped with highly-parallel x-ray beams (Gobel Mirror) and LynxEye detector. The samples were scanned from 3 to 40°2θ, at a step size 0.02°2θ and a time per step of 19.70 seconds. The tube voltage and current were 45 kV and 40 mA, respectively. The sample was transferred from sample container onto zero background XRD-holder and gently ground.
Syn
EuropeanJournalofMedicinalChemistry265(2024)116124
Vamorolone (Agamree)
On October 26, 2023, Vamorolone, developed jointly by Santhera Pharmaceuticals and ReveraGen BioPharma, has received FDA approval to treat DMD in patients aged 2 years and older [1]. DMD is a prevalent neuromuscular disorder in childhood, ranking among the most common.
This condition is caused by mutations in the gene responsible for producing the dystrophin protein, which plays a crucial role in maintaining muscle integrity. Moreover, DMD is an X-linked genetic disorder [69]. Vamorolone is a novel steroidal anti-inflammatory and membrane-stabilizing agent that can be taken orally. The distinction between it and traditional corticosteroid drugs lies in its capacity to
specifically activate particular signaling pathways of corticosteroids. In individuals diagnosed with DMD, the primary mechanism through which corticosteroid drugs exhibit their effectiveness is by exerting
anti-inflammatory effects. However, the secondary activities of corticosteroids can lead to adverse effects that impact the overall well-being of patients. Vamorolone has the ability to decrease the occurrence of
adverse effects while still preserving the therapeutic effectiveness of corticosteroids in individuals with DMD [70].
Preparation of Vamorolone is depicted in Scheme 19, which began with commercially available steroid 3 TR VAMO-001 [71]. Copper catalyzed addition of VAMO-001 with trimethylsilyl chloride (TMSCl)
gave silyl enol ether VAMO-002. VAMO-002 was oxidized by peracetic acid in acetic acid to yield intermediate VAMO-003, which was deprotected and hydrolyzed to obtain Vamorolone.
[69] D. Duan, N. Goemans, S. Takeda, E. Mercuri, A. Aartsma-Rus, Duchenne muscular
dystrophy, Nat. Rev. Dis. Prim. 7 (2021) 13.
[70] M. Guglieri, P.R. Clemens, S.J. Perlman, E.C. Smith, I. Horrocks, R.S. Finkel, J.
K. Mah, N. Deconinck, N. Goemans, J. Haberlova, V. Straub, L.J. Mengle-Gaw, B.
D. Schwartz, A.D. Harper, P.B. Shieh, L. De Waele, D. Castro, M.L. Yang, M.
M. Ryan, C.M. McDonald, M. Tulinius, R. Webster, H.J. McMillan, N.L. Kuntz, V.
K. Rao, G. Baranello, S. Spinty, A.M. Childs, A.M. Sbrocchi, K.A. Selby,
M. Monduy, Y. Nevo, J.J. Vilchez-Padilla, A. Nascimento-Osorio, E.H. Niks, I.J.
M. de Groot, M. Katsalouli, M.K. James, J. van den Anker, J.M. Damsker,
A. Ahmet, L.M. Ward, M. Jaros, P. Shale, U.J. Dang, E.P. Hoffman, Efficacy and
safety of vamorolone vs placebo and prednisone among boys with duchenne
muscular dystrophy: a randomized clinical trial, JAMA Neurol. 79 (2022)
1005–1014.
[71] E.K.M. Reeves, E.P. Hoffman, K. Nagaraju, J.M. Damsker, J.M. McCall, VBP15:
preclinical characterization of a novel anti-inflammatory delta 9,11 steroid,
Bioorg. Med. Chem. 21 (2013) 2241–2249.

Syn
J. Med. Chem. 2025, 68, 2147−2182
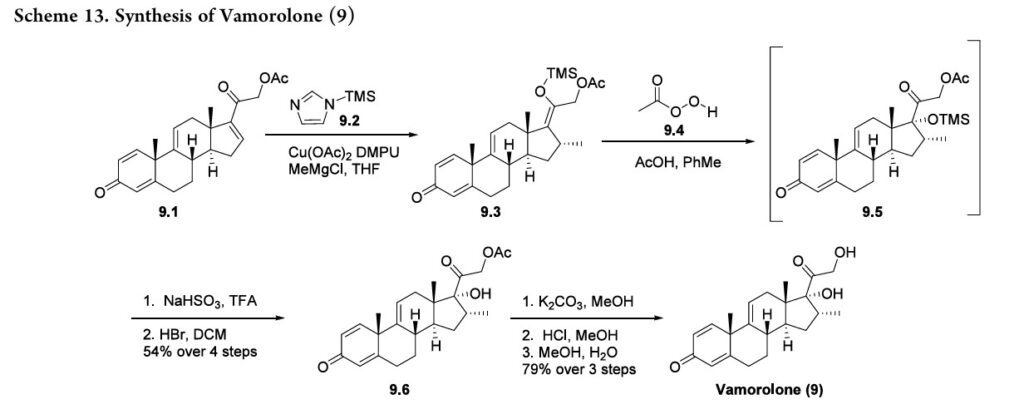
Vamorolone (Agamree). Developed by Santhera and ReveraGen BioPharma, the corticosteroid vamorolone (9) was approved for the treatment of Duchenne muscular dystrophy in October 2023.
70 Traditional corticosteroid treatment has been hampered by safety concerns including decreased bone mineral density and increased muscle atrophy. 71−73 Vamorolone is structurally distinct from other corticosteroids such as prednisone (Figure 3). 74 Removalofthe11βcarbonylmaintains binding to the glucocorticoid receptor but results in mineralocorticoid receptor antagonism; prednisone is a
mineralocorticoid receptor agonist. 75,76 This also results in decreased glucocorticoid receptor-drive transactivation, ultimately improving the safety profile of vamorolone as compared to other corticosteroid therapies. 74 The synthesis of vamorolone (9) as disclosed by ReveraGen BioPharma is summarized in Scheme 13. 77 Readily available steroid 9.1 was subjected to copper-catalyzed Michael addition.
Thein situ generated enolate was trapped using TMS-imidazole 9.2, providing the silyl enol ether 9.3. Treatment of crude 9.3 with peracetic acid 9.4 resulted in oxidized intermediate 9.5. Quenching of the peracetic acid and silyl deprotection afforded the protected steroid 9.6 in 54% yield from 9.1. Finally, K2CO3 mediated acetate deprotection of 9.6, neutralization and methanol/water crystallization provided vamorolone (9) in 79% yield over three steps.
(70) Keam, S. J. Vamorolone: first approval. Drugs 2024, 84, 111−
117.
(71) Hoffman, E. P.; Nader, G. A. Balancing muscle hypertrophy and
atrophy. Nat. Med. 2004, 10, 584−585.
(72) Hoffman, E. P.; Reeves, E.; Damsker, J.; Nagaraju, K.; McCall, J.
M.; Connor, E. M.; Bushby, K. Novel approaches to corticosteroid
treatment in Duchennemusculardystrophy.Phys.Med.Rehabil. Clin. N.
Am. 2012, 23, 821−828.
(73) Singh, A.; Schaeffer, E. K.; Reilly, C. W. Vertebral fractures in
Duchenne muscular dystrophy patients managed with Deflazacort. J.
Pediatr. Orthop. 2018, 38, 320−324.
(74) Liu, X.; Wang, Y.; Gutierrez, J. S.; Damsker, J. M.; Nagaraju, K.;
Hoffman, E. P.; Ortlund, E. A. Disruption of a key ligand-H-bond
network drives dissociative properties in vamorolone for Duchenne
muscular dystrophy treatment. Proc. Natl. Acad. Sci. U. S. A. 2020, 117,
24285−24293.
(75) Heier, C. R.; Yu, Q.; Fiorillo, A. A.; Tully, C. B.; Tucker, A.;
Mazala, D. A.; Uaesoontrachoon, K.; Srinivassane, S.; Damsker, J. M.;
Hoffman, E.P.; et al. Vamorolone targets dual nuclear receptors to treat
inflammation and dystrophic cardiomyopathy. Life Sci. Alliance 2019, 2,
No. e201800186.
(76)Boger, D.L.Thedifferenceasingleatomcanmake:synthesisand
design at the chemistry−biology interface. J. Org. Chem. 2017, 82,
11961−11980.
(77) Reeves, E. K. M.; Hoffman, E. P.; Nagaraju, K.; Damsker, J. M.;
McCall, J. M. VBP15: Preclinical characterization of a novel anti
inflammatory delta 9,11 steroid. Bioorg. Med. Chem. 2013, 21, 2241−
2249.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
PATENTS
Drugs 2024, 84, 111−117.
Nat. Med. 2004, 10, 584−585.
Clin. N.Am. 2012, 23, 821−828.
Pediatr. Orthop. 2018, 38, 320−324.
Proc. Natl. Acad. Sci. U. S. A. 2020, 117,24285−24293.
Life Sci. Alliance 2019, 2,No. e201800186.
J. Org. Chem. 2017, 82,11961−11980.
Bioorg. Med. Chem. 2013, 21, 2241−2249.
Neurol. 79 (2022)1005–1014
Bioorg. Med. Chem. 21 (2013) 2241–2249
Nat. Rev. Dis. Prim. 7 (2021) 13
Chemistry
Vamorolone is a synthetic corticosteroid and is also known by the chemical name 17α,21-dihydroxy-16α-methylpregna-1,4,9(11)-triene-3,20-dione or as 16α-methyl-9,11-dehydroprednisolone. It is a derivative of cortisol (hydrocortisone) and prednisolone (1,2-dehydrocortisol).
Anti-inflammatory drugs of the corticosteroid class show a carbonyl (=O) or hydroxyl (-OH) group on the C11 carbon of the steroid backbone. In contrast, vamorolone contains a Δ9,11 double bond between the C9 and C11 carbons. This change in structure has been shown to remove a molecular contact site with the glucocorticoid receptor, and leads to dissociative properties.[12]
History
In phase I clinical trials of adult volunteers, vamorolone was shown to be safe and well tolerated, with blood biomarker data suggesting possible loss of safety concerns of the corticosteroid class.[13]
In phase IIa dose-ranging clinical trial of 48 children with Duchenne muscular dystrophy (2 weeks on drug, 2 weeks off drug), vamorolone was shown to be safe and well tolerated, and showed blood biomarker data consistent with a myofiber membrane stabilization and anti-inflammatory effects, and possible loss of safety concerns.[14] These children continued on to a 24-week open-label extension study at the same doses, and this showed dose-dependent improvement of motor outcomes, with 2.0 and 6.0 mg/kg/day suggesting benefit.[15] These same children continued on a long-term extension study with dose escalations, and this suggested continued clinical improvement through 18-months treatment.[16]
Population pharmacokinetics (PK) of vamorolone was shown to fit to a 1-compartment model with zero-order absorption, with both adult men and young boys showing dose-linearity of PK parameters for the doses examined, and no accumulation of the drug during daily dosing. Apparent clearance averaged 2.0 L/h/kg in men and 1.7 L/h/kg in boys. Overall, vamorolone exhibited well-behaved linear PK, with similar profiles in healthy men and boys with DMD, moderate variability in PK parameters, and absorption and disposition profiles similar to those of classical glucocorticoids.[17] Exposure/response analyses have suggested that the motor outcome of time to stand from supine velocity showed the highest sensitivity to vamorolone, with the lowest AUC value providing 50% of maximum effect (E50 = 186 ng·h/mL), followed by time to climb 4 stairs (E50 = 478 ng·h/mL), time to run/walk 10 m (E50 = 1220 ng·h/mL), and 6-minute walk test (E50 = 1770 ng·h/mL). Week 2 changes of proinflammatory PD biomarkers showed exposure-dependent decreases. The E50 was 260 ng·h/mL for insulin-like growth factor-binding protein 2, 1200 ng·h/mL for matrix metalloproteinase 12, 1260 ng·h/mL for lymphotoxin α1/β2, 1340 ng·h/mL for CD23, 1420 ng·h/mL for interleukin-22-binding protein, and 1600 ng·h/mL for macrophage-derived chemokine/C-C motif chemokine 22.[18]
A trial titled “Efficacy and Safety of Vamorolone Over 48 Weeks in Boys With Duchenne Muscular Dystrophy” published in March 2024 found vamorolone (Agamree) at a dose of 6 mg/kg/d showed maintenance of improvement for all motor outcomes to week 48. There was also significant improvement in linear growth after crossover in the prednisone to vamorolone 6 mg/kg/d group, and quick reversal of prednisone-induced decline in bone turnover biomarkers in each crossover group.[19]
The US Food and Drug Administration (FDA) approved vamorolone based on evidence from a single clinical trial of 121 boys with DMD who were 4 to <7 years of age. The trial (Study 1) was conducted at 33 sites in 11 countries in Australia, Belgium, Canada, the Czech Republic, Spain, the United Kingdom, Greece, Israel, Netherlands, Sweden, and the United States.[10] In addition to Study 1, safety was also evaluated in a separate, open-label study of children with DMD aged 2 to <4 years (N=16) and children with DMD aged 7 to <18 years (N=16).[10]
Society and culture
Legal status
Santhera Pharmaceuticals signed an agreement with Catalyst Pharmaceuticals for the North American commercialization of vamorolone in July 2023.[20]
In October 2023, the FDA approved vamorolone (Agamree; Catalyst Pharmaceuticals) for the treatment of Duchenne muscular dystrophy.[11][21][22]
In October 2023, the Committee for Medicinal Products for Human Use adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Agamree, intended for the treatment of Duchenne muscular dystrophy.[2] The applicant for this medicinal product is Santhera Pharmaceuticals (Deutschland) GmbH.[2] Vamorolone was approved for medical use in the European Union in December 2023.[2][3]
Brand names
Vamorolone is the international nonproprietary name.[23]
Vamorolone is sold under the brand name Agamree.[1][2][3] Agamree (vamorolone) is a dissociative steroid that selectively binds to the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. Vamorolone also inhibits mineralocorticoid receptor activation by aldosterone.[24]
References
- “Agamree- vamorolone kit”. DailyMed. 26 October 2023. Retrieved 20 November 2023.
- “Agamree EPAR”. European Medicines Agency. 12 October 2023. Retrieved 27 December 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- “Agamree Product information”. Union Register of medicinal products. 15 December 2023. Retrieved 26 December 2023.
- “Vamorolone – ReveraGen Biopharma”. AdisInsight. Springer Nature Switzerland AG. Archived from the original on 7 October 2017. Retrieved 2 July 2017.
- Reeves EK, Hoffman EP, Nagaraju K, Damsker JM, McCall JM (April 2013). “VBP15: preclinical characterization of a novel anti-inflammatory delta 9,11 steroid”. Bioorganic & Medicinal Chemistry. 21 (8): 2241–2249. doi:10.1016/j.bmc.2013.02.009. PMC 4088988. PMID 23498916.
- Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, et al. (October 2013). “VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects”. EMBO Molecular Medicine. 5 (10): 1569–1585. doi:10.1002/emmm.201302621. PMC 3799580. PMID 24014378.
- Dadgar S, Wang Z, Johnston H, Kesari A, Nagaraju K, Chen YW, et al. (October 2014). “Asynchronous remodeling is a driver of failed regeneration in Duchenne muscular dystrophy”. The Journal of Cell Biology. 207 (1): 139–158. doi:10.1083/jcb.201402079. PMC 4195829. PMID 25313409.
- Damsker JM, Conklin LS, Sadri S, Dillingham BC, Panchapakesan K, Heier CR, et al. (September 2016). “VBP15, a novel dissociative steroid compound, reduces NFκB-induced expression of inflammatory cytokines in vitro and symptoms of murine trinitrobenzene sulfonic acid-induced colitis”. Inflammation Research. 65 (9): 737–743. doi:10.1007/s00011-016-0956-8. PMID 27261270. S2CID 18698831.
- Heier CR, Yu Q, Fiorillo AA, Tully CB, Tucker A, Mazala DA, et al. (February 2019). “Vamorolone targets dual nuclear receptors to treat inflammation and dystrophic cardiomyopathy”. Life Sci Alliance. 2 (1): e201800186. doi:10.26508/lsa.201800186. PMC 6371196. PMID 30745312.
- “Drug Trials Snapshots: Agamree”. U.S. Food and Drug Administration (FDA). 16 February 2024. Archived from the original on 18 February 2024. Retrieved 14 March 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - “Drug Approval Package: Agamree”. U.S. Food and Drug Administration (FDA). 7 November 2023. Archived from the original on 13 November 2023. Retrieved 13 November 2023.
- Liu X, Wang Y, Gutierrez JS, Damsker JM, Nagaraju K, Hoffman EP, et al. (September 2020). “Disruption of a key ligand-H-bond network drives dissociative properties in vamorolone for Duchenne muscular dystrophy treatment”. Proceedings of the National Academy of Sciences of the United States of America. 117 (39): 24285–24293. Bibcode:2020PNAS..11724285L. doi:10.1073/pnas.2006890117. PMC 7533876. PMID 32917814.
- Hoffman EP, Riddle V, Siegler MA, Dickerson D, Backonja M, Kramer WG, et al. (June 2018). “Phase 1 trial of vamorolone, a first-in-class steroid, shows improvements in side effects via biomarkers bridged to clinical outcomes”. Steroids. 134: 43–52. doi:10.1016/j.steroids.2018.02.010. PMC 6136660. PMID 29524454.
- Conklin LS, Damsker JM, Hoffman EP, Jusko WJ, Mavroudis PD, Schwartz BD, et al. (October 2018). “Phase IIa trial in Duchenne muscular dystrophy shows vamorolone is a first-in-class dissociative steroidal anti-inflammatory drug”. Pharmacological Research. 136: 140–150. doi:10.1016/j.phrs.2018.09.007. PMC 6218284. PMID 30219580.
- Hoffman EP, Schwartz BD, Mengle-Gaw LJ, Smith EC, Castro D, Mah JK, et al. (September 2019). “Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function”. Neurology. 93 (13): e1312 – e1323. doi:10.1212/WNL.0000000000008168. PMC 7011869. PMID 31451516.
- Smith EC, Conklin LS, Hoffman EP, Clemens PR, Mah JK, Finkel RS, et al. (September 2020). “Efficacy and safety of vamorolone in Duchenne muscular dystrophy: An 18-month interim analysis of a non-randomized open-label extension study”. PLOS Medicine. 17 (9): e1003222. doi:10.1371/journal.pmed.1003222. PMC 7505441. PMID 32956407.
- Mavroudis PD, van den Anker J, Conklin LS, Damsker JM, Hoffman EP, Nagaraju K, et al. (July 2019). “Population Pharmacokinetics of Vamorolone (VBP15) in Healthy Men and Boys With Duchenne Muscular Dystrophy”. Journal of Clinical Pharmacology. 59 (7): 979–988. doi:10.1002/jcph.1388. PMC 6548694. PMID 30742306.
- Li X, Conklin LS, van den Anker J, Hoffman EP, Clemens PR, Jusko WJ (October 2020). “Exposure-Response Analysis of Vamorolone (VBP15) in Boys With Duchenne Muscular Dystrophy”. Journal of Clinical Pharmacology. 60 (10): 1385–1396. doi:10.1002/jcph.1632. PMC 7494537. PMID 32434278.
- “Efficacy and Safety of Vamorolone Over 48 Weeks in Boys With Duchenne Muscular Dystrophy: A Randomized Controlled Trial”. PMID 38335499.
{{cite web}}: Missing or empty|url=(help) - Deswal P. “Santhera and Catalyst to market DMD drug vamorolone in North America”. Pharmaceutical Technology.
- “FDA Approves Vamorolone for Treatment of Duchenne Muscular Dystrophy in Patients Aged 2 Years and Older”. Pharmacy Times. 26 October 2023. Archived from the original on 27 October 2023. Retrieved 27 October 2023.
- “Santhera Receives U.S. FDA Approval of Agamree (vamorolone) for the Treatment of Duchenne Muscular Dystrophy” (Press release). Santhera Pharmaceuticals Holding AG. 27 October 2023. Archived from the original on 31 October 2023. Retrieved 13 November 2023 – via GlobeNewswire.
- World Health Organization (2017). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 77”. WHO Drug Information. 31 (1). hdl:10665/330984.
- “Agamree for the Treatment of Duchenne Muscular Dystrophy, US”. Clinicaltrials Arena. Retrieved 11 February 2025.
External links
Clinical trial number NCT03439670 for “A Study to Assess the Efficacy and Safety of Vamorolone in Boys With Duchenne Muscular Dystrophy (DMD)” at ClinicalTrials.gov
- [1]. Heier CR, et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. 2013 Oct;5(10):1569-85. [Content Brief][2]. Dillingham BC, et al. VBP15, a novel anti-inflammatory, is effective at reducing the severity of murine experimental autoimmune encephalomyelitis. Cell Mol Neurobiol. 2015 Apr;35(3):377-387. [Content Brief][3]. Heier CR, et al. Vamorolone targets dual nuclear receptors to treat inflammation and dystrophic cardiomyopathy. Life Sci Alliance. 2019 Feb 11;2(1). pii: e201800186. [Content Brief]
| Clinical data | |
|---|---|
| Trade names | Agamree |
| Other names | VBP; VBP-15; 17α,21-Dihydroxy-16α-methylpregna-1,4,9(11)-triene-3,20-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a624005 |
| License data | US DailyMed: Vamorolone |
| Routes of administration | By mouth |
| ATC code | H02AB18 (WHO) |
| Legal status | |
| Legal status | US: ℞-only[1]EU: Rx-only[2][3] |
| Identifiers | |
| IUPAC name | |
| CAS Number | 13209-41-1 |
| PubChem CID | 3035000 |
| DrugBank | DB15114 |
| ChemSpider | 2299335 |
| UNII | 8XP29XMB43 |
| KEGG | D11000 |
| ChEBI | CHEBI:228304 |
| ChEMBL | ChEMBL2348780 |
| CompTox Dashboard (EPA) | DTXSID60927527 |
| ECHA InfoCard | 100.032.874 |
| Chemical and physical data | |
| Formula | C22H28O4 |
| Molar mass | 356.462 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
////////////Vamorolone, VBP 15, APPROVALS 2023, EMA 2023, FDA 2023, 8XP29XMB43, AGAMREE, EU 2023














